Diverse role of NMDA receptors for dendritic integration of neural dynamics
- PMID: 37036844
- PMCID: PMC10085026
- DOI: 10.1371/journal.pcbi.1011019
Diverse role of NMDA receptors for dendritic integration of neural dynamics
Abstract
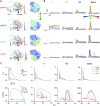
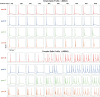
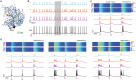
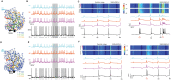
Neurons, represented as a tree structure of morphology, have various distinguished branches of dendrites. Different types of synaptic receptors distributed over dendrites are responsible for receiving inputs from other neurons. NMDA receptors (NMDARs) are expressed as excitatory units, and play a key physiological role in synaptic function. Although NMDARs are widely expressed in most types of neurons, they play a different role in the cerebellar Purkinje cells (PCs). Utilizing a computational PC model with detailed dendritic morphology, we explored the role of NMDARs at different parts of dendritic branches and regions. We found somatic responses can switch from silent, to simple spikes and complex spikes, depending on specific dendritic branches. Detailed examination of the dendrites regarding their diameters and distance to soma revealed diverse response patterns, yet explain two firing modes, simple and complex spike. Taken together, these results suggest that NMDARs play an important role in controlling excitability sensitivity while taking into account the factor of dendritic properties. Given the complexity of neural morphology varying in cell types, our work suggests that the functional role of NMDARs is not stereotyped but highly interwoven with local properties of neuronal structure.
Copyright: © 2023 Tang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses.J Neurophysiol. 1994 Jan;71(1):401-19. doi: 10.1152/jn.1994.71.1.401. J Neurophysiol. 1994. PMID: 8158238
-
Modification of Synaptic-Input Clustering by Intrinsic Excitability Plasticity on Cerebellar Purkinje Cell Dendrites.J Neurosci. 2020 Jan 8;40(2):267-282. doi: 10.1523/JNEUROSCI.3211-18.2019. Epub 2019 Nov 21. J Neurosci. 2020. PMID: 31754008 Free PMC article.
-
Long-Lasting Somatic Modifications of Convergent Dendritic Inputs in Hippocampal Neurons.Cereb Cortex. 2020 Mar 14;30(3):1436-1446. doi: 10.1093/cercor/bhz177. Cereb Cortex. 2020. PMID: 31504279
-
Synaptic integration in dendritic trees.J Neurobiol. 2005 Jul;64(1):75-90. doi: 10.1002/neu.20144. J Neurobiol. 2005. PMID: 15884003 Review.
-
The decade of the dendritic NMDA spike.J Neurosci Res. 2010 Nov 1;88(14):2991-3001. doi: 10.1002/jnr.22444. J Neurosci Res. 2010. PMID: 20544831 Free PMC article. Review.
Cited by
-
What makes human cortical pyramidal neurons functionally complex.bioRxiv [Preprint]. 2024 Dec 19:2024.12.17.628883. doi: 10.1101/2024.12.17.628883. bioRxiv. 2024. PMID: 39763809 Free PMC article. Preprint.
-
Dentate gyrus granule cell activation following extracellular electrical stimulation: a multi-scale computational model to guide hippocampal neurostimulation strategies.Front Comput Neurosci. 2025 Aug 1;19:1638002. doi: 10.3389/fncom.2025.1638002. eCollection 2025. Front Comput Neurosci. 2025. PMID: 40822710 Free PMC article.
References
-
- Stuart G, Spruston N, Häusser M. Dendrites. Oxford University Press; 2016.

