ENRICHing medical imaging training sets enables more efficient machine learning
- PMID: 37036945
- PMCID: PMC10198519
- DOI: 10.1093/jamia/ocad055
ENRICHing medical imaging training sets enables more efficient machine learning
Abstract
Objective: Deep learning (DL) has been applied in proofs of concept across biomedical imaging, including across modalities and medical specialties. Labeled data are critical to training and testing DL models, but human expert labelers are limited. In addition, DL traditionally requires copious training data, which is computationally expensive to process and iterate over. Consequently, it is useful to prioritize using those images that are most likely to improve a model's performance, a practice known as instance selection. The challenge is determining how best to prioritize. It is natural to prefer straightforward, robust, quantitative metrics as the basis for prioritization for instance selection. However, in current practice, such metrics are not tailored to, and almost never used for, image datasets.
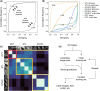
Materials and methods: To address this problem, we introduce ENRICH-Eliminate Noise and Redundancy for Imaging Challenges-a customizable method that prioritizes images based on how much diversity each image adds to the training set.
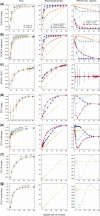
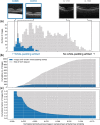
Results: First, we show that medical datasets are special in that in general each image adds less diversity than in nonmedical datasets. Next, we demonstrate that ENRICH achieves nearly maximal performance on classification and segmentation tasks on several medical image datasets using only a fraction of the available images and without up-front data labeling. ENRICH outperforms random image selection, the negative control. Finally, we show that ENRICH can also be used to identify errors and outliers in imaging datasets.
Conclusions: ENRICH is a simple, computationally efficient method for prioritizing images for expert labeling and use in DL.
Keywords: data efficiency; data quality; deep learning; information theory; instance selection; medical imaging.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures
References
-
- Lee G, Fujita H, eds. Deep Learning in Medical Image Analysis: Challenges and Applications. Switzerland: Springer International Publishing; 2020. doi:10.1007/978-3-030-33128-3. - DOI

