Lipid droplets are intracellular mechanical stressors that impair hepatocyte function
- PMID: 37036981
- PMCID: PMC10120019
- DOI: 10.1073/pnas.2216811120
Lipid droplets are intracellular mechanical stressors that impair hepatocyte function
Abstract
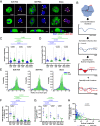
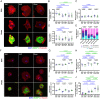
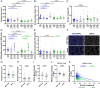
Matrix stiffening and external mechanical stress have been linked to disease and cancer development in multiple tissues, including the liver, where cirrhosis (which increases stiffness markedly) is the major risk factor for hepatocellular carcinoma. Patients with nonalcoholic fatty liver disease and lipid droplet-filled hepatocytes, however, can develop cancer in noncirrhotic, relatively soft tissue. Here, by treating primary human hepatocytes with the monounsaturated fatty acid oleate, we show that lipid droplets are intracellular mechanical stressors with similar effects to tissue stiffening, including nuclear deformation, chromatin condensation, and impaired hepatocyte function. Mathematical modeling of lipid droplets as inclusions that have only mechanical interactions with other cellular components generated results consistent with our experiments. These data show that lipid droplets are intracellular sources of mechanical stress and suggest that nuclear membrane tension integrates cell responses to combined internal and external stresses.
Keywords: HNF4α; chromatin condensation; cytoskeleton; mechanobiology; nuclear deformation.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Lipid droplets disrupt mechanosensing in human hepatocytes.Am J Physiol Gastrointest Liver Physiol. 2020 Jul 1;319(1):G11-G22. doi: 10.1152/ajpgi.00098.2020. Epub 2020 May 28. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32463334 Free PMC article.
-
Deranged hepatocyte intracellular Ca2+ homeostasis and the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma.Cell Calcium. 2019 Sep;82:102057. doi: 10.1016/j.ceca.2019.102057. Epub 2019 Jul 26. Cell Calcium. 2019. PMID: 31401389 Review.
-
Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes.Mol Metab. 2021 May;47:101168. doi: 10.1016/j.molmet.2021.101168. Epub 2021 Jan 16. Mol Metab. 2021. PMID: 33465519 Free PMC article.
-
Biomechanics of cultured hepatic cells during different steatogenic hits.J Mech Behav Biomed Mater. 2019 Sep;97:296-305. doi: 10.1016/j.jmbbm.2019.05.036. Epub 2019 May 22. J Mech Behav Biomed Mater. 2019. PMID: 31151002
-
Perspectives on Mitochondria-ER and Mitochondria-Lipid Droplet Contact in Hepatocytes and Hepatic Lipid Metabolism.Cells. 2021 Sep 1;10(9):2273. doi: 10.3390/cells10092273. Cells. 2021. PMID: 34571924 Free PMC article. Review.
Cited by
-
The triglyceride-synthesizing enzyme diacylglycerol acyltransferase 2 modulates the formation of the hepatitis C virus replication organelle.PLoS Pathog. 2024 Sep 6;20(9):e1012509. doi: 10.1371/journal.ppat.1012509. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39241103 Free PMC article.
-
Measuring age-dependent viscoelasticity of organelles, cells and organisms with time-shared optical tweezer microrheology.Nat Nanotechnol. 2025 Mar;20(3):411-420. doi: 10.1038/s41565-024-01830-y. Epub 2025 Jan 2. Nat Nanotechnol. 2025. PMID: 39747604 Free PMC article.
-
Mechanobiology of portal hypertension.JHEP Rep. 2023 Aug 2;5(11):100869. doi: 10.1016/j.jhepr.2023.100869. eCollection 2023 Nov. JHEP Rep. 2023. PMID: 37841641 Free PMC article. Review.
-
The rheology and interfacial properties of biomolecular condensates.Biophys Rev. 2025 Jun 30;17(3):867-891. doi: 10.1007/s12551-025-01326-6. eCollection 2025 Jun. Biophys Rev. 2025. PMID: 40727669 Free PMC article. Review.
-
Elongation factor 1A1 inhibition elicits changes in lipid droplet size, the bulk transcriptome, and cell type-associated gene expression in MASLD mouse liver.Am J Physiol Gastrointest Liver Physiol. 2024 Oct 1;327(4):G608-G622. doi: 10.1152/ajpgi.00276.2023. Epub 2024 Aug 13. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 39136056 Free PMC article.
References
-
- Paik J. M., Golabi P., Younossi Y., Mishra A., Younossi Z. M., Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD. Hepatology 72, 1605–1616 (2020). - PubMed
-
- Farrell A., Ryan M., Howell J., Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A western perspective. Hepatoma Res. 6, 18 (2020).
-
- Villanueva A., Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019). - PubMed
-
- Nahon P., et al. , Liver stiffness measurement in patients with cirrhosis and hepatocellular carcinoma: A case-control study. Eur. J. Gastroenterol. Hepatol. 21, 214–219 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

