Impact of extended interval dosing of immune checkpoint inhibitors in lung cancer patients during the COVID-19 pandemic
- PMID: 37037058
- PMCID: PMC9937999
- DOI: 10.1016/j.resmer.2023.101004
Impact of extended interval dosing of immune checkpoint inhibitors in lung cancer patients during the COVID-19 pandemic
Abstract
Background: The COVID 19-pandemic has led physicians to change their approach to treating non-small cell lung cancer (NSCLC) to reduce hospital stays for patients.
Objectives: We aimed to assess the toxicity and efficacy of extended interval (EI) dosing of immune checkpoint inhibitors (ICIs) compared to standard dosing (SD).
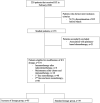
Methods: In this retrospective two-center study, we included patients with stage III/IV NSCLC who were treated with ICIs with or without maintenance pemetrexed during the month before March 2020. Adverse events and efficacy were collected until June 2021. Toxicity and survival were assessed using multivariate Cox models.
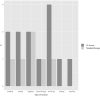
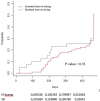
Results: Among the 134 patients identified (8 stage III and 126 stage IV; 66 first line and 60 second or subsequent lines), 70.9% received EI dosing. In the EI group, 12.6% of patients developed grade 3 or 4 immune-related adverse events versus 15.4% in the SD group (P- value = 0.8). Treatment was definitively discontinued due to toxicity in 9 patients in the EI group and in 5 in the SD group (P-value =0.5). Overall survival was not associated with dosage regimen or toxicity analyzed as a time-dependent variable.
Conclusions: Our study suggests that EI dosing of ICIs did not affect toxicity and overall survival in lung cancer patients.
Keywords: COVID 19-pandemic; Immune checkpoint inhibitors; Immune-related toxicity; Lung cancer.
Copyright © 2023 SPLF and Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflict of interest.
Figures
References
-
- Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han Ji-Y, Molina J, et al. « Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial ». Lancet North Am Ed. 2016;387(10027):1540‑50. napril. - PubMed
-
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Pawel J, Gadgeel SM, et al. « Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial ». Lancet North Am Ed. 2017;389(10066):255‑65. njanvier. - PMC - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, et al. « Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater ». J Clin Oncol. 2019;37(7):537‑46. n1 mars. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical