Elevated Tacrolimus Blood Concentration Due to the Interaction with Nirmatrelvir/Ritonavir During COVID-19 Treatment: A Case Report
- PMID: 37037726
- PMCID: PMC10027946
- DOI: 10.1016/j.transproceed.2023.03.001
Elevated Tacrolimus Blood Concentration Due to the Interaction with Nirmatrelvir/Ritonavir During COVID-19 Treatment: A Case Report
Abstract
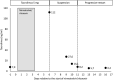
Nirmatrelvir/ritonavir, a newly authorized drug for the treatment of COVID-19, is a strong CYP3A4 inhibitor that can interact with many drugs, such as tacrolimus, reducing its metabolism. The reported case is a renal transplant patient who experienced a strong increase in tacrolimus blood concentration (up to 112ng/mL, more than ten times above the target range) when treated with both drugs at the same time, which caused a neurologic condition that required hospital admission for its control and treatment. The resolution of the symptoms was rapid, but the elevation of tacrolimus concentration remained for several days, even after discontinuing both drugs. This case shows the importance of developing a protocol for managing this interaction to guarantee the efficacy and safety of treatment with nirmatrelvir/ritonavir in transplant patients.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures
References
-
- Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther. 2011;90:666–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical