Diabetic vascular diseases: molecular mechanisms and therapeutic strategies
- PMID: 37037849
- PMCID: PMC10086073
- DOI: 10.1038/s41392-023-01400-z
Diabetic vascular diseases: molecular mechanisms and therapeutic strategies
Abstract
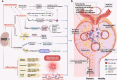
Vascular complications of diabetes pose a severe threat to human health. Prevention and treatment protocols based on a single vascular complication are no longer suitable for the long-term management of patients with diabetes. Diabetic panvascular disease (DPD) is a clinical syndrome in which vessels of various sizes, including macrovessels and microvessels in the cardiac, cerebral, renal, ophthalmic, and peripheral systems of patients with diabetes, develop atherosclerosis as a common pathology. Pathological manifestations of DPDs usually manifest macrovascular atherosclerosis, as well as microvascular endothelial function impairment, basement membrane thickening, and microthrombosis. Cardiac, cerebral, and peripheral microangiopathy coexist with microangiopathy, while renal and retinal are predominantly microangiopathic. The following associations exist between DPDs: numerous similar molecular mechanisms, and risk-predictive relationships between diseases. Aggressive glycemic control combined with early comprehensive vascular intervention is the key to prevention and treatment. In addition to the widely recommended metformin, glucagon-like peptide-1 agonist, and sodium-glucose cotransporter-2 inhibitors, for the latest molecular mechanisms, aldose reductase inhibitors, peroxisome proliferator-activated receptor-γ agonizts, glucokinases agonizts, mitochondrial energy modulators, etc. are under active development. DPDs are proposed for patients to obtain more systematic clinical care requires a comprehensive diabetes care center focusing on panvascular diseases. This would leverage the advantages of a cross-disciplinary approach to achieve better integration of the pathogenesis and therapeutic evidence. Such a strategy would confer more clinical benefits to patients and promote the comprehensive development of DPD as a discipline.
© 2023. The Author(s).
Conflict of interest statement
The authors report no commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

