The effect of barium and strontium on activity of glucoamylase QsGH97a from Qipengyuania seohaensis SW-135
- PMID: 37037863
- PMCID: PMC10086023
- DOI: 10.1038/s41598-023-32161-y
The effect of barium and strontium on activity of glucoamylase QsGH97a from Qipengyuania seohaensis SW-135
Abstract
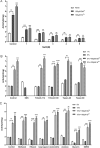
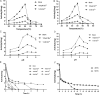
Glycoside hydrolases (GHs), the enzymes that break glycosidic bonds, are ubiquitous in the ecosystem, where they perform a range of biological functions. As an interesting glycosidase family, Glycoside hydrolase family 97 (GH97) contains α-glucosidase, α-galactosidase, and glucoamylase. Only ten members of GH97 have been characterized so far. It is critical to explore novel members to elucidate the catalytic mechanism and application potential of GH97 family. In this study, a novel glucoamylase QsGH97a from Qipengyuania seohaensis SW-135 was cloned and expressed in E. coli. Sequence analysis and NMR results show that QsGH97a is classified into GH97a, and adopts inverting mechanism. The biochemical characterization indicates that QsGH97a shows the optimal activity at 50 °C and pH 8.0. Ca2+ has little effect on the catalytic activity; however, the activity can be substantially increased by 8-13 folds in the presence of Ba2+ or Sr2+. Additionally, the metal content of QsGH97a assay showed a high proportion of Sr2+. The specific metal activity was initially revealed in glucoamylases, which is not found in other members. These results imply that QsGH97a not only is a new member of GH97, but also has potential for industrial applications. Our study reveals that Ba2+ or Sr2+ may be involved in the catalytic mechanism of glucoamylase, laying the groundwork for a more complete knowledge of GH97 and its possible industrial application.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Cai Y, Wang M, Xiao X, Liang B, Fan S, Zheng Z, Cosnier S, Liu A. A membraneless starch/O(2) biofuel cell based on bacterial surface regulable displayed sequential enzymes of glucoamylase and glucose dehydrogenase. Biosens. Bioelectron. 2022;207:114197. doi: 10.1016/j.bios.2022.114197. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

