An Integrative Multiomics Framework for Identification of Therapeutic Targets in Pulmonary Fibrosis
- PMID: 37038090
- PMCID: PMC10238219
- DOI: 10.1002/advs.202207454
An Integrative Multiomics Framework for Identification of Therapeutic Targets in Pulmonary Fibrosis
Abstract
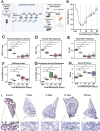
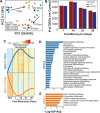
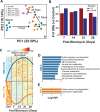
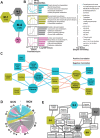
Pulmonary fibrosis (PF) is a heterogeneous disease with a poor prognosis. Therefore, identifying additional therapeutic modalities is required to improve outcome. However, the lack of biomarkers of disease progression hampers the preclinical to clinical translational process. Here, this work assesses and identifies progressive alterations in pulmonary function, transcriptomics, and metabolomics in the mouse lung at 7, 14, 21, and 28 days after a single dose of oropharyngeal bleomycin. By integrating multi-omics data, this work identifies two central gene subnetworks associated with multiple critical pathological changes in transcriptomics and metabolomics as well as pulmonary function. This work presents a multi-omics-based framework to establish a translational link between the bleomycin-induced PF model in mice and human idiopathic pulmonary fibrosis to identify druggable targets and test therapeutic candidates. This work also indicates peripheral cannabinoid receptor 1 (CB1 R) antagonism as a rational therapeutic target for clinical translation in PF. Mouse Lung Fibrosis Atlas can be accessed freely at https://niaaa.nih.gov/mouselungfibrosisatlas.
Keywords: CB1R; IPF; Irf5; bleomycin; metabolomics; mouse model; multi-omics; network biology; pulmonary fibrosis; systems biology; systems pharmacology; transcriptomics.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
