Viral predation pressure on coral reefs
- PMID: 37038111
- PMCID: PMC10088212
- DOI: 10.1186/s12915-023-01571-9
Viral predation pressure on coral reefs
Abstract
Background: Predation pressure and herbivory exert cascading effects on coral reef health and stability. However, the extent of these cascading effects can vary considerably across space and time. This variability is likely a result of the complex interactions between coral reefs' biotic and abiotic dimensions. A major biological component that has been poorly integrated into the reefs' trophic studies is the microbial community, despite its role in coral death and bleaching susceptibility. Viruses that infect bacteria can control microbial densities and may positively affect coral health by controlling microbialization. We hypothesize that viral predation of bacteria has analogous effects to the top-down pressure of macroorganisms on the trophic structure and reef health.
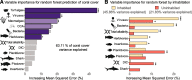
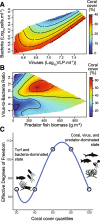
Results: Here, we investigated the relationships between live coral cover and viruses, bacteria, benthic algae, fish biomass, and water chemistry in 110 reefs spanning inhabited and uninhabited islands and atolls across the Pacific Ocean. Statistical learning showed that the abundance of turf algae, viruses, and bacteria, in that order, were the variables best predicting the variance in coral cover. While fish biomass was not a strong predictor of coral cover, the relationship between fish and corals became apparent when analyzed in the context of viral predation: high coral cover (> 50%) occurred on reefs with a combination of high predator fish biomass (sum of sharks and piscivores > 200 g m-2) and high virus-to-bacteria ratios (> 10), an indicator of viral predation pressure. However, these relationships were non-linear, with reefs at the higher and lower ends of the coral cover continuum displaying a narrow combination of abiotic and biotic variables, while reefs at intermediate coral cover showed a wider range of parameter combinations.
Conclusions: The results presented here support the hypothesis that viral predation of bacteria is associated with high coral cover and, thus, coral health and stability. We propose that combined predation pressures from fishes and viruses control energy fluxes, inhibiting the detrimental accumulation of ecosystem energy in the microbial food web.
Keywords: Bacteriophages; Benthic cover; Coral cover; Fish biomass; Microbialization; Phase-shift.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Moberg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol Econ. 1999;29:215–233. doi: 10.1016/S0921-8009(99)00009-9. - DOI
-
- Birkeland C. Life and death of coral reefs. New York: Springer New York; 1997.

