Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia
- PMID: 37038165
- PMCID: PMC10084658
- DOI: 10.1186/s12951-023-01874-7
Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia
Abstract
Background: Doxorubicin (Dox) has been recommended in clinical guidelines for the standard-of-care treatment of breast cancer. However, Dox therapy faces challenges such as hypoxia, acidosis, H2O2-rich conditions and condensed extracellular matrix in TME as well as low targeted ability.
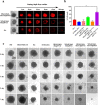
Methods: We developed a nanosystem H-MnO2-Dox-Col NPs based on mesoporous manganese dioxide (H-MnO2) in which Dox was loaded in the core and collagenase (Col) was wrapped in the surface. Further the H-MnO2-Dox-Col NPs were covered by a fusion membrane (MP) of inflammation-targeted RAW264.7 cell membrane and pH-sensitive liposomes to form biomimetic MP@H-MnO2-Dox-Col for in vitro and in vivo study.
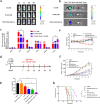
Results: Our results shows that MP@H-MnO2-Dox-Col can increase the Dox effect with low cardiotoxicity based on multi-functions of effective penetration in tumor tissue, alleviating hypoxia in TME, pH sensitive drug release as well as targeted delivery of Dox.
Conclusions: This multifunctional biomimetic nanodelivery system exhibited antitumor efficacy in vivo and in vitro, thus having potential for the treatment of breast cancer.
Keywords: Biomimetic nanoparticles; Breast cancer; Chemotherapy; Hypoxia; Penetration.
© 2023. The Author(s).
Conflict of interest statement
There is no conflict of interest to declare.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

