The cervical lymph node contributes to peripheral inflammation related to Parkinson's disease
- PMID: 37038192
- PMCID: PMC10088204
- DOI: 10.1186/s12974-023-02770-5
The cervical lymph node contributes to peripheral inflammation related to Parkinson's disease
Abstract
Background: Peripheral inflammation is an important feature of Parkinson's disease (PD). However, if and how CNS pathology is involved in the peripheral inflammation in PD remains to be fully investigated. Recently, the existence of meningeal lymphatics and its involvement in draining cerebral spinal fluid (CSF) to the cervical lymph node has been discovered. It is known that meningeal lymphatic dysfunction exists in idiopathic PD. The deep cervical lymph node (dCLN) substantially contributes to the drainage of the meningeal lymphatics. In addition, one of the lymphatics draining components, CSF, contains abundant α-synuclein (α-syn), a protein critically involved in PD pathogenesis and neuroinflammation. Thus, we began with exploring the possible structural and functional alterations of the dCLN in a PD mouse model (A53T mice) and investigated the role of pathological α-syn in peripheral inflammation and its potential underlying molecular mechanisms.
Methods: In this study, the transgenic mice (prnp-SNCA*A53T) which specifically overexpressed A53T mutant α-syn in CNS were employed as the PD animal model. Immunofluorescent and Hematoxylin and eosin staining were used to evaluate structure of dCLN. Inflammation in dCLNs as well as in bone-marrow-derived macrophages (BMDMs) was assessed quantitatively by measuring the mRNA and protein levels of typical inflammatory cytokines (including IL-1β, IL-6 and TNF-α). Intra-cisterna magna injection, flow cytometric sorting and electrochemiluminescence immunoassays were applied to investigate the lymphatic drainage of α-syn from the CNS. RNA-seq and Western blot were used to explore how pathological α-syn mediated the inflammation in PD mice.
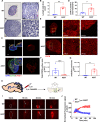
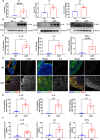
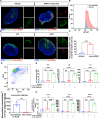
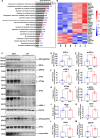
Results: The results unequivocally revealed substantially enlarged dCLNs, along with slow lymphatic flow, and increased inflammation in the dCLNs of A53T mice. Oligomeric α-syn drained from CSF potently activated macrophages in the dCLN via endoplasmic reticulum (ER) stress. Notably, inhibition of ER stress effectively suppressed peripheral inflammation in PD mice.
Conclusions: Our findings indicate that lymph node enlargement is closely related to macrophage activation, induced by meningeal lymphatics draining oligomeric α-syn, and contributes to the peripheral inflammation in PD. In addition, ER stress is a potential therapeutic target to ameliorate PD pathogenesis.
Keywords: Cervical lymph node; Endoplasmic reticulum stress; Inflammation; Macrophage; Parkinson’s disease; α-Synuclein.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Macrophages in the dCLN are required for α-synuclein proteostasis in A53T PD model mice.Brain Res. 2025 Aug 1;1860:149692. doi: 10.1016/j.brainres.2025.149692. Epub 2025 May 10. Brain Res. 2025. PMID: 40355037
-
Deep cervical lymph nodes in Parkinson's disease and atypical Parkinson's disease: A potential ultrasound biomarker for differential diagnosis.J Cent Nerv Syst Dis. 2024 Jul 29;16:11795735241259429. doi: 10.1177/11795735241259429. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 39086599 Free PMC article.
-
α-Synuclein-containing erythrocytic extracellular vesicles: essential contributors to hyperactivation of monocytes in Parkinson's disease.J Neuroinflammation. 2022 Feb 22;19(1):53. doi: 10.1186/s12974-022-02413-1. J Neuroinflammation. 2022. PMID: 35193594 Free PMC article.
-
The Role of α-Synuclein Oligomers in Parkinson's Disease.Int J Mol Sci. 2020 Nov 17;21(22):8645. doi: 10.3390/ijms21228645. Int J Mol Sci. 2020. PMID: 33212758 Free PMC article. Review.
-
α-Synuclein in Parkinson's disease: causal or bystander?J Neural Transm (Vienna). 2019 Jul;126(7):815-840. doi: 10.1007/s00702-019-02025-9. Epub 2019 Jun 25. J Neural Transm (Vienna). 2019. PMID: 31240402 Review.
Cited by
-
Characteristic fingerprint spectrum of α-synuclein mutants on terahertz time-domain spectroscopy.Biophys J. 2024 May 21;123(10):1264-1273. doi: 10.1016/j.bpj.2024.04.011. Epub 2024 Apr 16. Biophys J. 2024. PMID: 38615192 Free PMC article.
-
Association of glymphatic system function with peripheral inflammation and motor symptoms in Parkinson's disease.NPJ Parkinsons Dis. 2025 Mar 28;11(1):62. doi: 10.1038/s41531-025-00909-0. NPJ Parkinsons Dis. 2025. PMID: 40155401 Free PMC article.
-
Microbiota-gut-brain axis: interplay between microbiota, barrier function and lymphatic system.Gut Microbes. 2024 Jan-Dec;16(1):2387800. doi: 10.1080/19490976.2024.2387800. Epub 2024 Aug 25. Gut Microbes. 2024. PMID: 39182226 Free PMC article. Review.
-
Advancements in Genetic and Biochemical Insights: Unraveling the Etiopathogenesis of Neurodegeneration in Parkinson's Disease.Biomolecules. 2024 Jan 5;14(1):73. doi: 10.3390/biom14010073. Biomolecules. 2024. PMID: 38254673 Free PMC article. Review.
-
Integration of National Health Insurance claims data and animal models reveals fexofenadine as a promising repurposed drug for Parkinson's disease.J Neuroinflammation. 2024 Feb 21;21(1):53. doi: 10.1186/s12974-024-03041-7. J Neuroinflammation. 2024. PMID: 38383441 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

