Magnetic resonance imaging to assess the brain response to fasting in glioblastoma-bearing rats as a model of cancer anorexia
- PMID: 37038232
- PMCID: PMC10088192
- DOI: 10.1186/s40644-023-00553-y
Magnetic resonance imaging to assess the brain response to fasting in glioblastoma-bearing rats as a model of cancer anorexia
Abstract
Background: Global energy balance is a vital process tightly regulated by the brain that frequently becomes dysregulated during the development of cancer. Glioblastoma (GBM) is one of the most investigated malignancies, but its appetite-related disorders, like anorexia/cachexia symptoms, remain poorly understood.
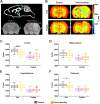
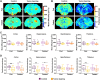
Methods: We performed manganese enhanced magnetic resonance imaging (MEMRI) and subsequent diffusion tensor imaging (DTI), in adult male GBM-bearing (n = 13) or control Wistar rats (n = 12). A generalized linear model approach was used to assess the effects of fasting in different brain regions involved in the regulation of the global energy metabolism: cortex, hippocampus, hypothalamus and thalamus. The regions were selected on the contralateral side in tumor-bearing animals, and on the left hemisphere in control rats. An additional DTI-only experiment was completed in two additional GBM (n = 5) or healthy cohorts (n = 6) to assess the effects of manganese infusion on diffusion measurements.
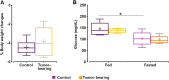
Results: MEMRI results showed lower T1 values in the cortex (p-value < 0.001) and thalamus (p-value < 0.05) of the fed ad libitum GBM animals, as compared to the control cohort, consistent with increased Mn2+ accumulation. No MEMRI-detectable differences were reported between fed or fasting rats, either in control or in the GBM group. In the MnCl2-infused cohorts, DTI studies showed no mean diffusivity (MD) variations from the fed to the fasted state in any animal cohort. However, the DTI-only set of acquisitions yielded remarkably decreased MD values after fasting only in the healthy control rats (p-value < 0.001), and in all regions, but thalamus, of GBM compared to control animals in the fed state (p-value < 0.01). Fractional anisotropy (FA) decreased in tumor-bearing rats due to the infiltrate nature of the tumor, which was detected in both diffusion sets, with (p-value < 0.01) and without Mn2+ administration (p-value < 0.001).
Conclusions: Our results revealed that an altered physiological brain response to fasting occurred in hunger related regions in GBM animals, detectable with DTI, but not with MEMRI acquisitions. Furthermore, the present results showed that Mn2+ induces neurotoxic inflammation, which interferes with diffusion MRI to detect appetite-induced responses through MD changes.
Keywords: Cancer anorexia; Diffusion tensor imaging; Fasting paradigm; Glioblastoma; Manganese enhanced MRI.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

