Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: a genome-wide study
- PMID: 37038246
- PMCID: PMC10232274
- DOI: 10.1093/eurheartj/ehad142
Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: a genome-wide study
Abstract
Aims: Although highly heritable, the genetic etiology of calcific aortic stenosis (AS) remains incompletely understood. The aim of this study was to discover novel genetic contributors to AS and to integrate functional, expression, and cross-phenotype data to identify mechanisms of AS.
Methods and results: A genome-wide meta-analysis of 11.6 million variants in 10 cohorts involving 653 867 European ancestry participants (13 765 cases) was performed. Seventeen loci were associated with AS at P ≤ 5 × 10-8, of which 15 replicated in an independent cohort of 90 828 participants (7111 cases), including CELSR2-SORT1, NLRP6, and SMC2. A genetic risk score comprised of the index variants was associated with AS [odds ratio (OR) per standard deviation, 1.31; 95% confidence interval (CI), 1.26-1.35; P = 2.7 × 10-51] and aortic valve calcium (OR per standard deviation, 1.22; 95% CI, 1.08-1.37; P = 1.4 × 10-3), after adjustment for known risk factors. A phenome-wide association study indicated multiple associations with coronary artery disease, apolipoprotein B, and triglycerides. Mendelian randomization supported a causal role for apolipoprotein B-containing lipoprotein particles in AS (OR per g/L of apolipoprotein B, 3.85; 95% CI, 2.90-5.12; P = 2.1 × 10-20) and replicated previous findings of causality for lipoprotein(a) (OR per natural logarithm, 1.20; 95% CI, 1.17-1.23; P = 4.8 × 10-73) and body mass index (OR per kg/m2, 1.07; 95% CI, 1.05-1.9; P = 1.9 × 10-12). Colocalization analyses using the GTEx database identified a role for differential expression of the genes LPA, SORT1, ACTR2, NOTCH4, IL6R, and FADS.
Conclusion: Dyslipidemia, inflammation, calcification, and adiposity play important roles in the etiology of AS, implicating novel treatments and prevention strategies.
Keywords: Aortic stenosis; Gene expression; Genetic risk score; Genome-wide association study; Mendelian randomization; Phenome-wide association study.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest Scott M. Damrauer receives research support (to the University of Pennsylvania) from RenalytixAI and personal fees from Caico Ibs, both outside the scope of the present work. SMD is also named as a co-inventor on a government-owned US Patent application related to the use of genetic risk prediction for venous thromboembolic disease filed by the US Department of Veterans Affairs in accordance with Federal regulatory requirements. SMD is named as a co-inventor on a Government-owned US Patent application related to the use of PDE3B inhibition for preventing cardiovascular disease filed by the US Department of Veterans Affairs in accordance with Federal regulatory requirements. Stefan Söderberg has received speaker honoraria and consulting fees from Actelion Ltd. George Thanassoulis has received consulting fees from Ionis Pharmaceuticals and has participated in advisory boards for Amgen, Sanofi, Novartis, HLS Therapeutics and Silence. Morten Salling Olesen has received 5.000.000 dkrfra Sundhedsdonationer.Journalnr. 2022-0243. David O. Arnar has received travel support from Pfizer to attend the ESC 2022 Scientific Meeting in Barcelona and has stock options in Sidekick Health Digital Therapeutics. Henning Bundgaard has received lecture fees from Amgen, MSD, Sanofi-Avensis, BMS and grants from NordForsk, Innovation Fond, Denmark, The Capital Regions Research Foundation. Alex Hoerby Christensen—Novo Nordisk Foundation NNF20OC0065799. Romaine Capoulade has received an Honorarium for one lecture from Novartis. Robert Clarke has received support from BAYER (China Kadoorie Biobank). Unnur Thorsteinsdottir’s research is funded by deCODE genetics/Amgen. Daniel F. Gudbjartsson receives funds from deCODE Genetics/Amgen. Until 1 June 2022, Gudmundur Thorgeirsson was a part time employee of deCode Genetics that is owned by Amgen. Hilma Holm is an employee of deCODE genetics/Amgen Inc. Anna Helgadottir is an employee of deCODE genetics/Amgen Inc.
Figures

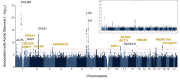
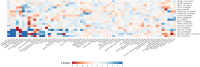
Comment in
-
The journey towards identification of actionable molecular pathways in calcific aortic valve stenosis.Eur Heart J. 2023 Jun 1;44(21):1940-1942. doi: 10.1093/eurheartj/ehad134. Eur Heart J. 2023. PMID: 37042229 No abstract available.
References
-
- Members WC, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, et al. . 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25–e197. 10.1016/j.jacc.2020.11.018 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 HC095168/HL/NHLBI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- S10 RR025141/RR/NCRR NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- U01 HG006378/HG/NHGRI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- R01 HL071739/HL/NHLBI NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- RE/18/3/34214/BHF_/British Heart Foundation/United Kingdom
- U19 HL065962/HL/NHLBI NIH HHS/United States
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 HL128550/HL/NHLBI NIH HHS/United States
- R01 HL146860/HL/NHLBI NIH HHS/United States
- R01 HD074711/HD/NICHD NIH HHS/United States
- IK2 CX001780/CX/CSRD VA/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- RC2 GM092618/GM/NIGMS NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- R01 NS032830/NS/NINDS NIH HHS/United States
- U01 HG004798/HG/NHGRI NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- P30 AG072972/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

