Total neoadjuvant therapy in rectal cancer: a network meta-analysis of randomized trials
- PMID: 37038270
- PMCID: PMC10475801
- DOI: 10.3393/ac.2022.00920.0131
Total neoadjuvant therapy in rectal cancer: a network meta-analysis of randomized trials
Abstract
Purpose: To assess the efficacy of total neoadjuvant therapy (TNT) for rectal carcinoma in comparison with conventional chemoradiotherapy (CRT).
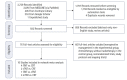
Methods: A systematic review was performed according to the PRISMA guidelines. A Bayesian network meta-analysis was done using NetMetaXL and WinBUGS. This study was registered in PROSPERO on March 3, 2022 (No. CRD-42022307867).
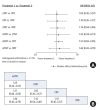
Results: Outcomes of 2,719 patients from 10 randomized trials between 2010 and 2022 were selected. Of these 1,191 (44%) had conventional long-course CRT (50-54 Gy) and capecitabine, 506 (18%) had induction chemotherapy followed by CRT (50-54 Gy) and capecitabine (iTNT), 230 (9%) had long-course CRT (50-54 Gy) followed by consolidation chemotherapy (cTNT), and 792 (29%) undergone modified short-course radiotherapy (25 Gy) with subsequent chemotherapy (mTNT). Total pathologic complete response (pCR) was 20% in the iTNT group, 21% in the mTNT group, 22% in the cTNT group, and 12% in the CRT group. Statistically significant difference in pCR rates was detected when comparing iTNT with CRT (odds ratio [OR], 1.76; 95% credible interval [CrI], 1.06-2.8), mTNT with CRT (OR, 1.90; 95% CrI, 1.25-2.74), and cTNT with CRT groups (OR, 2.54; 95% CrI, 1.26-5.08). No differences were found in R0 resection rates. No significant difference was found in long-term outcomes.
Conclusion: The early administration of systemic chemotherapy in the TNT regimen has improved short-term outcomes, though long-term results are underreported. Randomized trials with survival as the endpoint are necessary to evaluate the possible advantages of TNT modes.
Keywords: Colorectal disease; Neoadjuvant therapy; Network meta-analysis; Rectal neoplasms; Total neoadjuvant therapy.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures







References
-
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, RadosevicJelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23. - PubMed
-
- Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687–94. - PubMed
-
- Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822–32. - PubMed
-
- Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28. - PubMed
-
- Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

