Sputum pathogen spectrum and clinical outcomes of upper respiratory tract infection in bronchiectasis exacerbation: a prospective cohort study
- PMID: 37038356
- PMCID: PMC10167879
- DOI: 10.1080/22221751.2023.2202277
Sputum pathogen spectrum and clinical outcomes of upper respiratory tract infection in bronchiectasis exacerbation: a prospective cohort study
Abstract
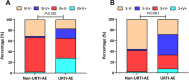
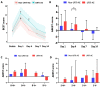
Upper respiratory tract infection (URTI) is common in humans. We sought to profile sputum pathogen spectrum and impact of URTI on acute exacerbation of bronchiectasis (AE). Between March 2017 and December 2021, we prospectively collected sputum from adults with bronchiectasis. We stratified AEs into events related (URTI-AE) and unrelated to URTI (non-URTI-AE). We captured URTI without onset of AE (URTI-non-AE). We did bacterial culture and viral detection with polymerase chain reaction, and explored the pathogen spectrum and clinical impacts of URTI-AE via longitudinal follow-up. Finally, we collected 479 non-AE samples (113 collected at URTI-non-AE and 225 collected at clinically stable) and 170 AE samples (89 collected at URTI-AE and 81 collect at non-URTI-AE). The viral detection rate was significantly higher in URTI-AE (46.1%) than in non-URTI-AE (4.9%) and URTI-non-AE (11.5%) (both P < 0.01). Rhinovirus [odds ratio (OR): 5.00, 95% confidence interval (95%CI): 1.06-23.56, P = 0.03] detection was independently associated with URTI-AE compared with non-URTI-AE. URTI-AE tended to yield higher viral load and detection rate of rhinovirus, metapneumovirus and bacterial shifting compared with URTI-non-AE. URTI-AE was associated with higher initial viral loads (esp. rhinovirus, metapneumovirus), greater symptom burden (higher scores of three validated questionnaires) and prolonged recovery compared to those without. Having experienced URTI-AE predicted a greater risk of future URTI-AE (OR: 10.90, 95%CI: 3.60-33.05). In summary, URTI is associated with a distinct pathogen spectrum and aggravates bronchiectasis exacerbation, providing the scientific rationale for the prevention of URTI to hinder bronchiectasis progression.
Keywords: Upper respiratory tract infections; bronchiectasis; exacerbation; symptom burden; virus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
[Respiratory pathogen spectrum in pulmonary exacerbation of bronchiectasis in adults and its association with disease severity].Zhonghua Jie He He Hu Xi Za Zhi. 2019 Apr 12;42(4):254-261. doi: 10.3760/cma.j.issn.1001-0939.2019.04.002. Zhonghua Jie He He Hu Xi Za Zhi. 2019. PMID: 30955282 Chinese.
-
The roles of bacteria and viruses in COPD-Bronchiectasis association: A prospective cohort study.Respir Med. 2024 Sep;231:107692. doi: 10.1016/j.rmed.2024.107692. Epub 2024 Jun 7. Respir Med. 2024. PMID: 38852923
-
Time-course of upper respiratory tract viral infection and COPD exacerbation.Eur Respir J. 2019 Oct 10;54(4):1900407. doi: 10.1183/13993003.00407-2019. Print 2019 Oct. Eur Respir J. 2019. PMID: 31391222
-
Efficacy and safety of long-term inhaled antibiotic for patients with noncystic fibrosis bronchiectasis: a meta-analysis.Clin Respir J. 2016 Nov;10(6):731-739. doi: 10.1111/crj.12278. Epub 2015 Mar 2. Clin Respir J. 2016. PMID: 25620629 Review.
-
Exercise training for bronchiectasis.Cochrane Database Syst Rev. 2021 Apr 6;4(4):CD013110. doi: 10.1002/14651858.CD013110.pub2. Cochrane Database Syst Rev. 2021. PMID: 33822364 Free PMC article.
Cited by
-
Bacteria and viruses and clinical outcomes of asthma-bronchiectasis overlap syndrome: A cohort study.Clin Transl Allergy. 2024 Jan;14(1):e12331. doi: 10.1002/clt2.12331. Clin Transl Allergy. 2024. PMID: 38282200 Free PMC article.
-
Effectiveness of Preprocedural Mouthwashes: A Triple-Blind Randomised Controlled Clinical Trial.Int Dent J. 2025 Apr;75(2):868-876. doi: 10.1016/j.identj.2024.08.017. Epub 2024 Oct 6. Int Dent J. 2025. PMID: 39370336 Free PMC article. Clinical Trial.
-
Infection and the microbiome in bronchiectasis.Eur Respir Rev. 2024 Jul 3;33(173):240038. doi: 10.1183/16000617.0038-2024. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 38960615 Free PMC article. Review.
-
Microbiological characteristics of the lower airway in adults with bronchiectasis: a prospective cohort study.Respir Res. 2024 Jul 17;25(1):283. doi: 10.1186/s12931-024-02903-1. Respir Res. 2024. PMID: 39020401 Free PMC article.
References
-
- Chang AB, Fortescue R, Grimwood K, et al. . European respiratory society guidelines for the management of children and adolescents with bronchiectasis. Eur Respir J. 2021;58(2):2002990. - PubMed
-
- Kapur N, Masters IB, Chang AB.. Exacerbations in noncystic fibrosis bronchiectasis: clinical features and investigations. Respir Med. 2009;103(11):1681–1687. - PubMed
-
- Chalmers JD, Aliberti S, Filonenko A, et al. . Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med. 2018;197(11):1410–1420. - PubMed
-
- Mac Aogain M, Narayana JK, Tiew PY, et al. . Integrative microbiomics in bronchiectasis exacerbations. Nat Med. 2021;27(4):688–699. - PubMed
-
- Dicker AJ, Lonergan M, Keir HR, et al. . The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med. 2021;9(8):885–896. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
