Inhalation Devices in 7- to 15-Year-Old Children with Asthma - A Patient Preference Study
- PMID: 37038436
- PMCID: PMC10082580
- DOI: 10.2147/PPA.S381486
Inhalation Devices in 7- to 15-Year-Old Children with Asthma - A Patient Preference Study
Abstract
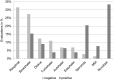
Background: Inhalation therapy is the cornerstone of treatment of bronchial asthma. A patient-specific selection of inhalation devices is necessary, as preference for a device plays an important role in terms of error rates in handling and adherence to therapy. However, there is no industry-independent study providing information on children's preferences for common inhaler types. The aim of the present study was to investigate the preference of asthmatic children for inhaler types commonly used in Germany. The effects of age, gender and the type of school visited on device preferences as well as the frequency of patient education and the role of health care providers in the choice for an inhaler were investigated.
Methods: Eighty children were included in this prospective cross-sectional study (age: 10.87 ± 2.62 years). The analysis was based on a questionnaire and validated checklists. All participants tested the use of nine placebo inhalers (Breezhaler, Diskus, Respimat, Spiromax, Turbohaler, Autohaler, metered-dose inhaler, Easyhaler and Novolizer) in a randomized order. For each device, patients were asked to assess handling, rate different device characteristics and name the device they would prefer most or least.
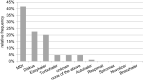
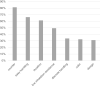
Results: The most favored device was the Novolizer. Moreover, the Spiromax scored highest in numerous categories such as suitability in emergencies and "easiest" device to use. Patient preferences with respect to the addressed inhaler features were not significantly related to age, gender or school type.
Conclusion: The Novolizer and the Spiromax showed higher preference in pediatric patients as compared to other tested devices. Overall, there were significant differences in terms of preference when comparing the tested inhalers in different aspects.
Keywords: childhood asthma; inhaler devices; inhaler technique; patient preferences.
© 2023 Tietz et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Inhaler devices in asthma and COPD patients - a prospective cross-sectional study on inhaler preferences and error rates.BMC Pulm Med. 2020 Aug 20;20(1):222. doi: 10.1186/s12890-020-01246-z. BMC Pulm Med. 2020. PMID: 32819337 Free PMC article.
-
Evaluation of inhaler handling-errors, inhaler perception and preference with Spiromax, Easyhaler and Turbuhaler devices among healthy Finnish volunteers: a single site, single visit crossover study (Finhaler).BMJ Open Respir Res. 2016 Mar 22;3(1):e000119. doi: 10.1136/bmjresp-2015-000119. eCollection 2016. BMJ Open Respir Res. 2016. PMID: 27026804 Free PMC article.
-
First-time handling of different inhalers by chronic obstructive lung disease patients.Exp Lung Res. 2020 Sep;46(7):258-269. doi: 10.1080/01902148.2020.1789903. Epub 2020 Jul 2. Exp Lung Res. 2020. PMID: 32614625 Clinical Trial.
-
How to achieve good compliance and adherence with inhalation therapy.Curr Med Res Opin. 2005;21 Suppl 4:S33-7. doi: 10.1185/030079905X61776. Curr Med Res Opin. 2005. PMID: 16138943 Review.
-
Requirements, Strengths and Weaknesses of Inhaler Devices for COPD Patients from the Expert Prescribers' Point of View: Results of the EPOCA Delphi Consensus.COPD. 2017 Dec;14(6):573-580. doi: 10.1080/15412555.2017.1365120. Epub 2017 Sep 11. COPD. 2017. PMID: 28891722
References
-
- World Health Organisation (WHO). Asthma: key facts; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma. Accessed March 24, 2022.
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2021. Available from: https://ginasthma.org/. Accessed March 24, 2022.
LinkOut - more resources
Full Text Sources

