Limited sampling strategies for individualized BAX 855 prophylaxis in severe hemophilia A: in silico evaluation
- PMID: 37038844
- PMCID: PMC10101132
- DOI: 10.1097/MBC.0000000000001204
Limited sampling strategies for individualized BAX 855 prophylaxis in severe hemophilia A: in silico evaluation
Abstract
Objective: Limited sampling strategies (LSS) lower the burden of pharmacokinetic (PK)-guided dosing, but an extensive evaluation of LSS for BAX 855 (Adynovi) is currently lacking. This study aimed to develop a LSS for BAX 855 and combine this with a LSS of a standard half-life (SHL) factor VIII (FVIII) concentrate in a clinical setting.
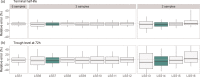
Methods: Individual PK parameters of BAX 855 were estimated for 10 000 virtual patients with severe hemophilia A using Monte Carlo simulations. Several LSS consisting of 2-6 samples were examined based on patient burden, bias and accuracy of clearance, elimination half-life, volume of distribution and trough levels at 72 h (C72). Analyses were performed separately for adults and children <12 years.
Results: The preferred LSS for BAX 855 consisted of three sampling points at 15-30 min, 48 h and 72 h for both adults (mean accuracy C72: 14.0% vs. 10.8% using six samples) and children (mean accuracy C72: 14.9% vs. 11.4% using six samples). The best strategy with two samples (peak, 48 h) resulted in an adequate, but lower accuracy than strategies with ≥3 samples (mean accuracy C72: 22.3%). The optimal combination of the LSS of SHL FVIII and BAX 855 led to six samples during four clinical visits.
Conclusion: This in silico study has identified that two to three samples are necessary to estimate the individual PK of BAX-855 adequately. These samples can be collected in one or two clinical visits. When combining PK profiling of SHL FVIII and BAX 855, six samples during four clinical visits are needed.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.H.C.'s institution has received investigator-initiated research and travel grants as well as speaker fees over the years from the Netherlands Organisation for Scientific Research (NWO) and Netherlands National research Agenda (NWA), the Netherlands Organization for Health Research and Development (ZonMw), the Dutch Innovatiefonds Zorgverzekeraars, Baxter/Baxalta/Shire/ Takeda, Pfizer, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis and Nordic Pharma, and for serving as a steering board member for Roche, Bayer and Novartis for which fees go to the Erasmus MC as an institution. R.A.A.M. has received grants from governmental and societal research institutes such as NWO, ZonMW, Dutch Kidney Foundation and Innovation Fund and unrestricted investigator research grants from Baxter/ Baxalta/ Shire/Takeda, Bayer, CSL Behring, Sobi and CelltrionHC. He has served as advisor for Bayer, CSL Behring, Merck Sharp & Dohme, Baxter/ Baxalta/ Shire/Takeda. All grants and fees paid to the institution. Other authors have no conflict of interest to declare for this paper.
Figures

References
-
- Manco-Johnson MJ, Abshire T, Shapiro AD, Riske BK, Hacker MR, Kilcoyne R, et al. . Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357:535–544. - PubMed
-
- Björkman S. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood 2012; 119:612–618. - PubMed
-
- Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. . WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26:1–158. - PubMed
-
- Lee M, Morfini M, Schulman S, Ingerslev J. The design and analysis of pharmacokinetic studies of coagulation factors. 2021. Available from: https://www.isth.org/members/group_content_view.asp?group=100348&id=159244. [Accessed 26 May 2021]
-
- Iorio A, Blanchette V, Blatny J, Collins P, Fischer K, Neufeld E. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost 2017; 15:2461–2465. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

