Irradiation stent with 125 I plus TACE versus sorafenib plus TACE for hepatocellular carcinoma with major portal vein tumor thrombosis: a multicenter randomized trial
- PMID: 37038986
- PMCID: PMC10389427
- DOI: 10.1097/JS9.0000000000000295
Irradiation stent with 125 I plus TACE versus sorafenib plus TACE for hepatocellular carcinoma with major portal vein tumor thrombosis: a multicenter randomized trial
Abstract
Background and aim: Treatment strategy for hepatocellular carcinoma (HCC) and Vp4 [main trunk] portal vein tumor thrombosis (PVTT) remains limited due to posttreatment liver failure. We aimed to assess the efficacy of irradiation stent placement with 125 I plus transcatheter arterial chemoembolization (TACE) (ISP-TACE) compared to sorafenib plus TACE (Sora-TACE) in these patients.
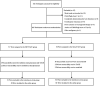
Methods: In this multicenter randomized controlled trial, participants with HCC and Vp4 PVTT without extrahepatic metastases were enrolled from November 2018 to July 2021 at 16 medical centers. The primary endpoint was overall survival (OS). The secondary endpoints were hepatic function, time to symptomatic progression, patency of portal vein, disease control rate, and treatment safety.
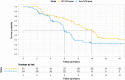
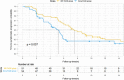
Results: Of 105 randomized participants, 51 were assigned to the ISP-TACE group, and 54 were assigned to the Sora-TACE group. The median OS was 9.9 months versus 6.3 months (95% CI: 0.27-0.82; P =0.01). Incidence of acute hepatic decompensation was 16% (8 of 51) versus 33% (18 of 54) ( P =0.036). The time to symptomatic progression was 6.6 months versus 4.2 months (95% CI: 0.38-0.93; P =0.037). The median stent patency was 7.2 months (interquartile range, 4.7-9.3) in the ISP-TACE group. The disease control rate was 86% (44 of 51) versus 67% (36 of 54) ( P =0.018). Incidences of adverse events at least grade 3 were comparable between the safety populations of the two groups: 16 of 49 (33%) versus 18 of 50 (36%) ( P =0.73).
Conclusion: Irradiation stent placement plus TACE showed superior results compared with sorafenib plus TACE in prolonging OS in patients with HCC and Vp4 PVTT.
Trial registration: ClinicalTrials.gov NCT03730675.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- European Association for the Study of the Liver. Electronic address easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. - PubMed
-
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–50. - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

