The Papain-Like Protease of Porcine Reproductive and Respiratory Syndrome Virus Impedes STING Translocation from the Endoplasmic Reticulum to the Golgi Apparatus by Deubiquitinating STIM1
- PMID: 37039642
- PMCID: PMC10134850
- DOI: 10.1128/jvi.00188-23
The Papain-Like Protease of Porcine Reproductive and Respiratory Syndrome Virus Impedes STING Translocation from the Endoplasmic Reticulum to the Golgi Apparatus by Deubiquitinating STIM1
Abstract
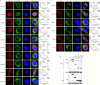
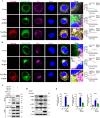
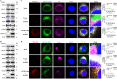
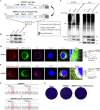
Stimulator of interferon (IFN) genes (STING) was recently pinpointed as an antiviral innate immune factor during the infection of RNA viruses. Porcine reproductive and respiratory syndrome virus (PRRSV), the swine arterivirus, is an enveloped RNA virus which has evolved many strategies to evade innate immunity. To date, the interactive network between PRRSV and STING remains to be fully established. Herein, we report that STING suppresses PRRSV replication through type I interferon signaling. However, PRRSV impedes STING trafficking from the endoplasmic reticulum (ER) to the Golgi apparatus, leading to the decreased phosphorylation of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3). Furthermore, PRRSV nonstructural protein 2 (Nsp2) colocalizes with STING, blocks STING translocation, and disrupts the STING-TBK1-IRF3 complex. Mechanistically, PRRSV Nsp2 retains STING at the ER by increasing the level of Ca2+ sensor stromal interaction molecule 1 (STIM1) protein. Functional analysis reveals that PRRSV Nsp2 deubiquitinates STIM1 by virtue of its papain-like protease 2 (PLP2) deubiquitinating (DUB) activity. Finally, we demonstrate that loss of STIM1 is associated with an elevated IFN response and restricts PRRSV replication. This work delineates the relationship between PRRSV infection and STING signaling and the importance of papain-like proteases (PLPs) in interfering in this axis. IMPORTANCE Porcine reproductive and respiratory syndrome virus (PRRSV), a member of the family Arteriviridae, is responsible for reproductive disorders in pregnant sows and respiratory problems in piglets, resulting in huge losses in the swine industry worldwide. Of note, PRRSV infection causes immunosuppression, of which the mechanism is not completely understood. Here, we demonstrate for the first time that STING, a protein typically associated with the antiviral response in DNA viruses, plays a critical role in controlling PRRSV infection. However, PRRSV utilizes its encoded protein Nsp2 to inhibit STING activity by blocking its translocation from the ER to the Golgi apparatus. In particular, Nsp2 retains STING at the ER by interacting with and further deubiquitinating STIM1. For this process, the activity of the viral PLP2 DUB enzyme is indispensable. The study describes a novel mechanism by which PLP2 plays a critical role in suppressing the innate immune response against arteriviruses and potentially other viruses that encode similar proteases.
Keywords: Nsp2; PRRSV; STIM1; STING; deubiquitinate; papain-like protease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kuhn JH, Lauck M, Bailey AL, Shchetinin AM, Vishnevskaya TV, Bào Y, Ng TFF, LeBreton M, Schneider BS, Gillis A, Tamoufe U, Diffo JLD, Takuo JM, Kondov NO, Coffey LL, Wolfe ND, Delwart E, Clawson AN, Postnikova E, Bollinger L, Lackemeyer MG, Radoshitzky SR, Palacios G, Wada J, Shevtsova ZV, Jahrling PB, Lapin BA, Deriabin PG, Dunowska M, Alkhovsky SV, Rogers J, Friedrich TC, O’Connor DH, Goldberg TL. 2016. Reorganization and expansion of the nidoviral family Arteriviridae. Arch Virol 161:755–768. doi: 10.1007/s00705-015-2672-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

