Comparison of a Rapid Multiplex Gastrointestinal Panel with Standard Laboratory Testing in the Management of Children with Hematochezia in a Pediatric Emergency Department: Randomized Controlled Trial
- PMID: 37039648
- PMCID: PMC10269456
- DOI: 10.1128/spectrum.00268-23
Comparison of a Rapid Multiplex Gastrointestinal Panel with Standard Laboratory Testing in the Management of Children with Hematochezia in a Pediatric Emergency Department: Randomized Controlled Trial
Abstract
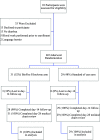
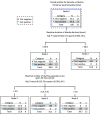
Advances in diagnostic microbiology allow for the rapid identification of a broad range of enteropathogens; such knowledge can inform care and reduce testing. We conducted a randomized, unblinded trial in a tertiary-care pediatric emergency department. Participants had stool (and rectal swabs if stool was not immediately available) tested using routine microbiologic approaches or by use of a device (BioFire FilmArray gastrointestinal panel), which identifies 22 pathogens with a 1-h instrument turnaround time. Participants were 6 months to <18.0 years and had acute bloody diarrhea. Primary outcome was performance of blood tests within 72 h. From 15 June 2018 through 7 May 2022, 60 children were randomized. Patients in the BioFire FilmArray arm had a reduced time to test result (median 3.0 h with interquartile range [IQR] of 3.0 to 4.0 h, versus 42.0 h (IQR 23.5 to 47.3 h); difference of -38.0 h, 95% confidence interval [CI] of -41.0 to -22.0 h). Sixty-five percent (20/31) of participants in the BioFire FilmArray group had a pathogen detected-most frequently enteropathogenic Escherichia coli (19%), Campylobacter (16%), and Salmonella (13%). Blood tests were performed in 52% of children in the BioFire FilmArray group and 62% in the standard-of-care group (difference of -10.5%, 95% CI of -35.4% to 14.5%). There were no between-group differences in the proportions of children administered intravenous fluids, antibiotics, hospitalized, or who had diagnostic imaging performed. Testing with the BioFire FilmArray reduced the time to result availability by 38 h. Although statistical significance was limited by study power, BioFire FilmArray use was not associated with clinically meaningful reductions in health care utilization or improved outcomes. IMPORTANCE Advances in diagnostic microbiology now allow for the faster and more accurate detection of an increasing number of pathogens. We determined, however, that in children with acute bloody diarrhea, these advances did not necessarily translate into improved clinical outcomes. While a greater number of pathogens was identified using a rapid turnaround multiplex stool diagnostic panel, with a reduction in the time to stool test result of over 1.5 days, this did not alter the practice of pediatric emergency medicine physicians, who continued to perform blood tests on a large proportion of children. While our conclusions may be limited by the relatively small sample size, targeted approaches that educate clinicians on the implementation of such technology into clinical care will be needed to optimize usage and maximize benefits.
Keywords: children; emergency department; hematochezia; randomized controlled trial; rapid multiplex gastrointestinal panel.
Conflict of interest statement
The authors declare a conflict of interest. A BioFire FilmArray Gastrointestinal Panel device was initially provided free of charge by bioMérieux. During the study period, a device was purchased for research purposes by the Alberta Children's Hospital Research Institute. All study test kits were purchased for usage.
Figures
References
-
- Böhrer M, Fitzpatrick E, Hurley K, Xie J, Lee BE, Pang X-L, Zhuo R, Parsons BD, Berenger BM, Chui L, Tarr PI, Ali S, Vanderkooi OG, Freedman SB, Zemek R, Newton M, Meckler G, Poonai N, Bhatt M, Maki K, McGahern C, Emerton R, The Alberta Provincial Pediatric EnTeric Infection TEam (APPETITE), Pediatric Emergency Research Canada (PERC) . 2022. Hematochezia in children with acute diarrhea seeking emergency department care: a prospective cohort study. Acad Emerg Med 29:429–441. doi:10.1111/acem.14434. - DOI - PubMed
-
- Böhrer M, Fitzpatrick E, Hurley K, et al. 2021. Hematochezia in children with acute diarrhea seeking emergency department care: a prospective cohort study. Acad Emerg Med. - PubMed
-
- McKee RS, Schnadower D, Tarr PI, Xie J, Finkelstein Y, Desai N, Lane RD, Bergmann KR, Kaplan RL, Hariharan S, Cruz AT, Cohen DM, Dixon A, Ramgopal S, Rominger A, Powell EC, Kilgar J, Michelson KA, Beer D, Bitzan M, Pruitt CM, Yen K, Meckler GD, Plint AC, Bradin S, Abramo TJ, Gouin S, Kam AJ, Schuh A, Balamuth F, Hunley TE, Kanegaye JT, Jones NE, Avva U, Porter R, Fein DM, Louie JP, Freedman SB. 2020. Predicting hemolytic uremic syndrome and renal replacement therapy in Shiga toxin-producing Escherichia coli-infected children. Clin Infect Dis 70:1643–1651. doi:10.1093/cid/ciz432. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

