Human Sapovirus Replication in Human Intestinal Enteroids
- PMID: 37039654
- PMCID: PMC10134857
- DOI: 10.1128/jvi.00383-23
Human Sapovirus Replication in Human Intestinal Enteroids
Abstract
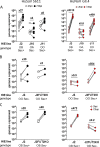
Human sapoviruses (HuSaVs), like human noroviruses (HuNoV), belong to the Caliciviridae family and cause acute gastroenteritis in humans. Since their discovery in 1976, numerous attempts to grow HuSaVs in vitro were unsuccessful until 2020, when these viruses were reported to replicate in a duodenal cancer cell-derived line. Physiological cellular models allowing viral replication are essential to investigate HuSaV biology and replication mechanisms such as genetic susceptibility, restriction factors, and immune responses to infection. In this study, we demonstrate replication of two HuSaV strains in human intestinal enteroids (HIEs) known to support the replication of HuNoV and other human enteric viruses. HuSaVs replicated in differentiated HIEs originating from jejunum, duodenum and ileum, but not from the colon, and bile acids were required. Between 2h and 3 to 6 days postinfection, viral RNA levels increased up from 0.5 to 1.8 log10-fold. Importantly, HuSaVs were able to replicate in HIEs independent of their secretor status and histo-blood group antigen expression. The HIE model supports HuSaV replication and allows a better understanding of host-pathogen mechanisms such as cellular tropism and mechanisms of viral replication. IMPORTANCE Human sapoviruses (HuSaVs) are a frequent but overlooked cause of acute gastroenteritis, especially in children. Little is known about this pathogen, whose successful in vitro cultivation was reported only recently, in a cancer cell-derived line. Here, we assessed the replication of HuSaV in human intestinal enteroids (HIEs), which are nontransformed cultures originally derived from human intestinal stem cells that can be grown in vitro and are known to allow the replication of other enteric viruses. Successful infection of HIEs with two strains belonging to different genotypes of the virus allowed discovery that the tropism of these HuSaVs is restricted to the small intestine, does not occur in the colon, and replication requires bile acid but is independent of the expression of histo-blood group antigens. Thus, HIEs represent a physiologically relevant model to further investigate HuSaV biology and a suitable platform for the future development of vaccines and antivirals.
Keywords: enteric virus; enteric viruses; histo-blood group antigens; human intestinal enteroids; norovirus; sapovirus.
Conflict of interest statement
M.K.E. is named as an inventor on patents related to cloning of the Norwalk virus genome and HuNoV cultivation and has received research funding from Takeda Vaccines Business Unit (Cambridge, MA, USA). R.L.A. is named as an inventor on patents related to HuNoV cultivation and has received research support from Takeda Vaccines Business Unit (Cambridge, MA, USA). The funders had no role in the study design; data collection, analyses, or interpretation; manuscript writing, or decision to publish the results so there are no competing interests. The remaining authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

