Quiet wakefulness: the influence of intraperitoneal and intranasal oxytocin on sleep-wake behavior and neurophysiology in rats
- PMID: 37039749
- PMCID: PMC10334738
- DOI: 10.1093/sleep/zsad112
Quiet wakefulness: the influence of intraperitoneal and intranasal oxytocin on sleep-wake behavior and neurophysiology in rats
Abstract
Study objectives: Exogenous administration of the neuropeptide oxytocin exerts diverse effects on various neurobehavioral processes, including sleep and wakefulness. Since oxytocin can enhance attention to social and fear-related environmental cues, it should promote arousal and wakefulness. However, as oxytocin can attenuate stress, reduce activity, and elicit anxiolysis, oxytocin might also prime the brain for rest, and promote sleep. At present, little research has comprehensively characterized the neuropsychopharmacology of oxytocin-induced effects on sleep-wake behavior and no reconciliation of these two competing hypotheses has been proposed.
Methods: This study explored the effects of oxytocin on sleep-wake outcomes using radiotelemetry-based polysomnography in adult male and female Wistar rats. Oxytocin was administered via intraperitoneal (i.p.; 0.1, 0.3 and 1 mg·kg-1) and intranasal (i.n.; 0.06, 1, 3 mg·kg-1) routes. Caffeine (i.p. and i.n.; 10 mg·kg-1) was administered as a wake-promoting positive control. To ascertain mechanism of action, pretreatment experiments with the oxytocin receptor (OXTR) antagonist L-368,899 (i.p.; 5 mg·kg-1) followed by oxytocin (i.p.; 1 mg·kg-1) were also conducted.
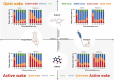
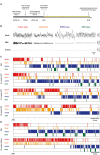
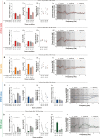
Results: In both male and female rats, i.p. oxytocin promoted quiet wakefulness at the cost of suppressing active wakefulness, non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. Several i.p. oxytocin-induced sleep-wake effects were mediated by OXTR binding. In contrast, i.n. oxytocin did not alter most sleep-wake outcomes at any dose tested. Both i.p. and i.n. caffeine demonstrated wake-promoting effects.
Conclusions: These findings help reconcile competing hypotheses of oxytocin-induced effects on sleep-wake behavior: i.p. oxytocin promotes quiet wakefulness-a state of restful environmental awareness compatible with both oxytocin's anxiolytic effects and its enhancement of processing complex stimuli.
Keywords: caffeine; intranasal; intraperitoneal; oxytocin; oxytocin receptor; quiet wakefulness; rat; rodent; sleep; wake.
© Sleep Research Society 2023. Published by Oxford University Press on behalf of the Sleep Research Society.
Figures




References
-
- Grinevich V, Ludwig M.. The multiple faces of the oxytocin and vasopressin systems in the brain. J Neuroendocrinol. 2021;33(11):e13004. - PubMed

