Activity-dependent post-translational regulation of palmitoylating and depalmitoylating enzymes in the hippocampus
- PMID: 37039765
- PMCID: PMC10113885
- DOI: 10.1242/jcs.260629
Activity-dependent post-translational regulation of palmitoylating and depalmitoylating enzymes in the hippocampus
Abstract
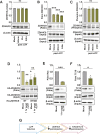
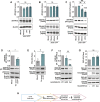
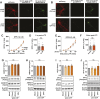
Activity-induced changes in protein palmitoylation can regulate the plasticity of synaptic connections, critically impacting learning and memory. Palmitoylation is a reversible post-translational modification regulated by both palmitoyl-acyl transferases that mediate palmitoylation and palmitoyl thioesterases that depalmitoylate proteins. However, it is not clear how fluctuations in synaptic activity can mediate the dynamic palmitoylation of neuronal proteins. Using primary hippocampal cultures, we demonstrate that synaptic activity does not impact the transcription of palmitoylating and depalmitoylating enzymes, changes in thioesterase activity, or post-translational modification of the depalmitoylating enzymes of the ABHD17 family and APT2 (also known as LYPLA2). In contrast, synaptic activity does mediate post-translational modification of the palmitoylating enzymes ZDHHC2, ZDHHC5 and ZDHHC9 (but not ZDHHC8) to influence protein-protein interactions, enzyme stability and enzyme function. Post-translational modifications of the ZDHHC enzymes were also observed in the hippocampus following fear conditioning. Taken together, our findings demonstrate that signaling events activated by synaptic activity largely impact activity of the ZDHHC family of palmitoyl-acyl transferases with less influence on the activity of palmitoyl thioesterases.
Keywords: Chemical long-term potentiation; Palmitoylation; Post-translational modification; Synapse activity; ZDHHC enzymes.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

