What Is the Efficacy of Intra-articular Injections in the Treatment of Ankle Osteoarthritis? A Systematic Review
- PMID: 37039814
- PMCID: PMC10427070
- DOI: 10.1097/CORR.0000000000002624
What Is the Efficacy of Intra-articular Injections in the Treatment of Ankle Osteoarthritis? A Systematic Review
Abstract
Background: Ankle osteoarthritis (OA) is painful and can impact a patient's physical and mental quality of life. Although intra-articular injections are commonly used to alleviate symptoms, there is conflicting evidence concerning their efficacy. Therefore, an updated systematic review would be informative.
Question/purpose: In this systematic review, we asked: Are there clinically important benefits or harms associated with the use of intra-articular injections in the treatment of ankle OA?
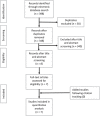
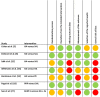
Methods: We used PubMed, Embase, and the Cochrane Library to search for randomized controlled trials on intra-articular injections for the treatment of ankle OA in June 2021, and updated the search in January 2022; eligible dates were from the date of inception of each database through January 2022. Reference lists of eligible studies and previous reviews were manually screened. Two reviewers independently assessed studies for eligibility. We included seven studies. Three compared hyaluronic acid (HA) with saline, one compared HA with exercise, one compared four different regimens of HA [ 34 ], one compared platelet-rich plasma (PRP) with saline, and one compared botulinum toxin Type A (BoNT-A) with HA. A total of 340 patients were included: 141 in the HA arms, 48 in the PRP arm, 38 in the BoNT-A arm, and 113 in the saline arms. Across all studies, the mean age was 52 ± 21 years, and 35% were women (119 of 340 patients). Methodologic quality was assessed using the Cochrane Risk of Bias 2.0 tool. Of the included studies, the risk of bias was low in two studies, presented some concerns in one study, and was high in four studies. According to the Grading of Recommendations Assessment, Development, and Evaluation methodology, the level of evidence was very low for HA, moderate for PRP, and very low for BoNT-A. The level of heterogeneity was high, and we opted to perform a systematic review rather than a meta-analysis. A clinically relevant difference was based on whether the between-group difference surpassed the cutoff point determined as the minimum clinically important difference.
Results: No clinically relevant differences were found among HA, PRP, and BoNT-A and their control groups at 3, 6, or 12 months. No studies reported any serious adverse events in any treatment group.
Conclusion: Given the lack of observed efficacy in this systematic review, these treatments should not be used in practice until or unless future high-quality studies find evidence of efficacy.
Level of evidence: Level III, therapeutic study.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Association of Bone and Joint Surgeons.
Conflict of interest statement
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members. All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures
Comment in
-
CORR Insights®: What Is the Efficacy of Intra-articular Injections in the Treatment of Ankle Osteoarthritis? A Systematic Review.Clin Orthop Relat Res. 2023 Sep 1;481(9):1825-1827. doi: 10.1097/CORR.0000000000002656. Epub 2023 Apr 11. Clin Orthop Relat Res. 2023. PMID: 37043554 Free PMC article. No abstract available.
Similar articles
-
Evidence on ankle injections for osteochondral lesions and osteoarthritis: a systematic review and meta-analysis.Int Orthop. 2021 Feb;45(2):509-523. doi: 10.1007/s00264-020-04689-5. Epub 2020 Jul 9. Int Orthop. 2021. PMID: 32647968
-
Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2021 Jan;49(1):249-260. doi: 10.1177/0363546520909397. Epub 2020 Apr 17. Am J Sports Med. 2021. PMID: 32302218
-
Platelet-Rich Plasma Combined With Hyaluronic Acid Improves Pain and Function Compared With Hyaluronic Acid Alone in Knee Osteoarthritis: A Systematic Review and Meta-analysis.Arthroscopy. 2021 Apr;37(4):1277-1287.e1. doi: 10.1016/j.arthro.2020.11.052. Epub 2020 Dec 3. Arthroscopy. 2021. PMID: 33278533
-
Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis.BMC Musculoskelet Disord. 2020 Apr 11;21(1):224. doi: 10.1186/s12891-020-03262-w. BMC Musculoskelet Disord. 2020. PMID: 32278352 Free PMC article.
-
Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis.Am J Sports Med. 2017 Feb;45(2):339-346. doi: 10.1177/0363546516665809. Epub 2016 Oct 21. Am J Sports Med. 2017. PMID: 28146403 Clinical Trial.
Cited by
-
Nanomaterial-Based Drug Delivery Systems Targeting Functional Cells for Osteoarthritis Treatment: Mechanisms, Challenges and Future Prospects.Int J Nanomedicine. 2025 Apr 25;20:5291-5320. doi: 10.2147/IJN.S518935. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40303574 Free PMC article. Review.
References
-
- Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785-793. - PubMed
-
- Boffa A, Previtali D, Di Laura Frattura G, Vannini F, Candrian C, Filardo G. Evidence on ankle injections for osteochondral lesions and osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2021;45:509-523. - PubMed
-
- Coe MP, Sutherland JM, Penner MJ, Younger A, Wing KJ. Minimal clinically important difference and the effect of clinical variables on the Ankle Osteoarthritis Scale in surgically treated end-stage ankle arthritis. J Bone Joint Surg Am. 2015;97:818-823. - PubMed
-
- Cohen J. A power primer. Psychol Bull. 1992;112:155-159. - PubMed
-
- Cohen MM, Altman RD, Hollstrom R, Hollstrom C, Sun C, Gipson B. Safety and efficacy of intra-articular sodium hyaluronate (Hyalgan®) in a randomized, double-blind study for osteoarthritis of the ankle. Foot Ankle Int. 2008;29:657-663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

