Molecular profiling and treatment pattern differences between intrahepatic and extrahepatic cholangiocarcinoma
- PMID: 37040087
- PMCID: PMC10323903
- DOI: 10.1093/jnci/djad046
Molecular profiling and treatment pattern differences between intrahepatic and extrahepatic cholangiocarcinoma
Abstract
Background: Treatment patterns for intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) differ, but limited studies exist comparing them. This study examines differences in molecular profiling rates and treatment patterns in these populations, focusing on use of adjuvant, liver-directed, targeted, and investigational therapies.
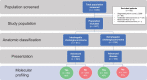
Methods: This multicenter collaboration included patients with ICC or ECC treated at 1 of 8 participating institutions. Retrospective data were collected on risk factors, pathology, treatments, and survival. Comparative statistical tests were 2-sided.
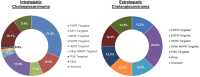
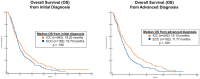
Results: Among 1039 patients screened, 847 patients met eligibility (ICC = 611, ECC = 236). Patients with ECC were more likely than those with ICC to present with early stage disease (53.8% vs 28.0%), undergo surgical resection (55.1% vs 29.8%), and receive adjuvant chemoradiation (36.5% vs 4.2%) (all P < .00001). However, they were less likely to undergo molecular profiling (50.3% vs 64.3%) or receive liver-directed therapy (17.9% vs 35.7%), targeted therapy (4.7% vs 18.9%), and clinical trial therapy (10.6% vs 24.8%) (all P < .001). In patients with recurrent ECC after surgery, the molecular profiling rate was 64.5%. Patients with advanced ECC had a shorter median overall survival than those with advanced ICC (11.8 vs 15.1 months; P < .001).
Conclusions: Patients with advanced ECC have low rates of molecular profiling, possibly in part because of insufficient tissue. They also have low rates of targeted therapy use and clinical trial enrollment. While these rates are higher in advanced ICC, the prognosis for both subtypes of cholangiocarcinoma remains poor, and a pressing need exists for new effective targeted therapies and broader access to clinical trials.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
KS reports advisory/consultancy: QED Therapeutics, Helsinn, the Lynx Group, and Caris Life Sciences.
LP no disclosures. IB no disclosures. JM no disclosures. AGB no disclosures. KZ no disclosures. AJ no disclosures. ADD no disclosures. SR no disclosures. TML no disclosures.
OER reports research support from Merck. Speaker for activities supported by educational grants from BMS and Merck. Consultant for Merck, Celgene, Five Prime, GSK, Bayer, Roche/Genentech, Puretech, Imvax, Sobi, Boehringer Ingelheim. In addition, Dr Rahma has patent “Methods of using pembrolizumab and trebananib” pending.
JS no disclosures. TTD no disclosures. MR no disclosures.
MLBP institutional funding from Taiho, AstraZeneca, Exelixis, BeiGene, Berg, Merck, Bayer, Nucana, Lilly, and Helsinn Therapeutics; personal consulting fees from Agios. MLBP reports National Cancer Institute (K08CA248473).
AXZ reports advisory/consultancy: Sanofi-aventis, Lilly, Merck, Eisai, Sirtex. Employment: I-MAB Biopharma.
JKL no disclosures.
JKL reports National Institutes of Health (NIH) (R37 CA225655).
AJI no disclosures. KB no disclosures. CV no disclosures.
LRR reports research funding (to their institution) from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest Inc, RedHill Biopharma, TARGET PharmaSolutions, and QED Therapeutics; and service on scientific advisory committees or as a consultant for AstraZeneca, Bayer, Eisai, Exact Sciences, Gilead Sciences, Global Life Science Consulting, GRAIL Inc, Hepion, MedEd Design LLC, Medscape, Novartis Venture Fund, Pontifax, Roche, and The Lynx Group.
SL no disclosures. NH no disclosures.
LWG reports advisory/consultancy: QED, Genentech, Merck, AstraZeneca, Exelixis, Boehringer Ingelheim. Research Funding: BMS, Agios, ASLAN, BeiGene, Basilea, Merck.
KM reports Stock and Other Ownership Interests: CytoDyn; Consulting or Advisory Role: Celgene, Genentech/Roche, AstraZeneca, Ipsen, Boston Scientific, Incyte (Inst), QED Therapeutics; Research Funding: FibroGen (Inst), Senhwa Biosciences (Inst), MedImmune (Inst), Agios (Inst), ArQule (Inst), Taiho Pharmaceutical (Inst), Gritstone Bio (Inst), Incyte (Inst), Merck (Inst), Vyriad (Inst), Turnstone Bio (Inst), AstraZeneca (Inst), Basilea (Inst).
MB no relevant disclosures.
RTS reports funding from Bayer, Bristol-Myers Squibb, Exelixis, IMV Inc, Loxo, Novocure, NUCANA, Pieris, QED Therapeutics, Rafael Pharmaceuticals, Seagen, and Taiho.
honoraria for serving on scientific advisory committees or as a consultant from AstraZeneca, Boehringer Ingelheim Pharma, CAMI, Clovis, Genentech, Incyte, Merck, QED Therapeutics, Servier, Taiho, Zymeworks, and Syros.
RKK reports research funding (to institution) from Agios, Astra Zeneca, Bayer, BMS, Eli Lilly, EMD Serono, Exelixis, Genentech/Roche, Loxo Oncology, Merck, Novartis, Partner Therapeutics, QED, Relay Therapeutics, Surface Oncology, Taiho; honoraria for serving on scientific advisory committees or as a consultant from Agios, Astra Zeneca, Exelixis, Ipsen, Merck (to institution) and from Exact Sciences, Gilead, and Kinnate (to self).
MMJ reports research funding (to institution) from Merck, EMD Serono, Novartis, Eli Lilly, Astra Zeneca, Genentech, Transthera, Meclun, BMS, Incyte, QED, Taiho, Servier, Oncosil, Basilea, Nucana and to self or as advisory board/DSMB member from Incyte, Zymeworks, Mundi Pharma, Nucana, MORE health and Origimed.
LG reports research funding (to their institution) from Adaptimmune, Bayer, Merck, Macrogenics, Genentech, Novartis, Incyte, Loxo Oncology, Relay Therapeutics, QED, Taiho Oncology, Leap Therapeutics, Bristol Myers Squibb, Nucana, and Servier; honoraria (to self) for serving on scientific advisory committees or as a consultant from Alentis Therapeutics AG, Black Diamond, Basilea, Genentech, Exelixis, Kinnate, H3Biomedicine, Incyte Corporation, QED Therapeutics, Sirtex Medical Ltd, The Servier Group, SIRTEX, Taiho Oncology, TranstheraBio, and participation on data safety monitoring boards for AstraZeneca. LG receives funding from the American Cancer Society Clinical Scientist Development Grant 134013‐CSDG‐19‐163‐01‐TBG, the NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003, V Foundation for Cancer Research Translational Grant, and the Cholangiocarcinoma Foundation Andrea Marie Fuquay Research Fellowship.
Figures
References
-
- Shaib YH, Davila JA, McGlynn K, et al.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472-477. - PubMed
-
- Khan SA, Emadossadaty S, Ladep NG, et al.Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848-854. - PubMed
-
- Welzel TM, McGlynn KA, Hsing AW, et al.Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98(12):873-875. - PubMed
-
- Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38(2):241-256. - PubMed

