Lecanemab for Patients With Early Alzheimer Disease: Bayesian Analysis of a Phase 2b Dose-Finding Randomized Clinical Trial
- PMID: 37040116
- PMCID: PMC10091161
- DOI: 10.1001/jamanetworkopen.2023.7230
Lecanemab for Patients With Early Alzheimer Disease: Bayesian Analysis of a Phase 2b Dose-Finding Randomized Clinical Trial
Abstract
Importance: Bayesian clinical trial designs are increasingly common; given their promotion by the US Food and Drug Administration, the future use of the bayesian approach will only continue to increase. Innovations possible when using the bayesian approach improve the efficiency of drug development and the accuracy of clinical trials, especially in the context of substantial data missingness.
Objective: To explain the foundations, interpretations, and scientific justification of the bayesian approach in the setting of lecanemab trial 201, a bayesian-designed phase 2 dose-finding trial; to demonstrate the efficiency of using a bayesian design; and to show how it accommodates innovations in the prospective design and also treatment-dependent types of missing data.
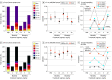
Design, setting, and participants: This study was a bayesian analysis of a clinical trial comparing the efficacy of 5 lecanemab 201 dosages for treatment of early Alzheimer disease. The goal of the lecanemab 201 trial was to identify the effective dose 90 (ED90), the dose achieving at least 90% of the maximum effectiveness of doses considered in the trial. This study assessed the bayesian adaptive randomization used, in which patients were preferentially assigned to doses that would give more information about the ED90 and its efficacy.
Interventions: Patients in the lecanemab 201 trial were adaptively randomized to 1 of 5 dose regimens or placebo.
Main outcomes and measures: The primary end point of lecanemab 201 was the Alzheimer Disease Composite Clinical Score (ADCOMS) at 12 months with continued treatment and follow-up out to 18 months.
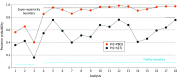
Results: A total 854 patients were included in trial treatment: 238 were in the placebo group (median age, 72 years [range, 50-89 years]; 137 female [58%]) and 587 were assigned to a lecanemab 201 treatment group (median age, 72 years [range, 50-90 years]; 272 female [46%]). The bayesian approach improved the efficiency of a clinical trial by prospectively adapting to the trial's interim results. By the trial's end more patients had been assigned to the better-performing doses: 253 (30%) and 161 (19%) patients to 10 mg/kg monthly and 10 mg/kg biweekly vs 51 (6%), 52 (6%), and 92 (11%) patients to 5 mg/kg monthly, 2.5 mg/kg biweekly, and 5 mg/kg biweekly, respectively. The trial identified 10 mg/kg biweekly as the ED90. The change in ADCOMS of the ED90 vs placebo was -0.037 at 12 months and -0.047 at 18 months. The bayesian posterior probability that the ED90 was superior to placebo was 97.5% at 12 months and 97.7% at 18 months. The respective probabilities of super-superiority were 63.8% and 76.0%. The primary analysis of the randomized bayesian lecanemab 201 trial found in the context of missing data that the most effective dose of lecanemab nearly doubles its estimated efficacy at 18 months of follow-up in comparison with restricting analysis to patients who completed the full 18 months of the trial.
Conclusions and relevance: Innovations associated with the bayesian approach can improve the efficiency of drug development and the accuracy of clinical trials, even in the context of substantial data missingness.
Trial registration: ClinicalTrials.gov Identifier: NCT01767311.
Conflict of interest statement
Figures