Combination of AFP vaccine and immune checkpoint inhibitors slows hepatocellular carcinoma progression in preclinical models
- PMID: 37040183
- PMCID: PMC10231990
- DOI: 10.1172/JCI163291
Combination of AFP vaccine and immune checkpoint inhibitors slows hepatocellular carcinoma progression in preclinical models
Abstract
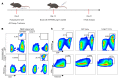
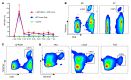
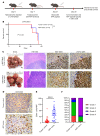
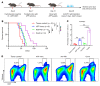
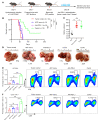
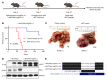
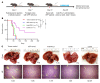
Many patients with hepatocellular carcinoma (HCC) do not respond to the first-line immune checkpoint inhibitor treatment. Immunization with effective cancer vaccines is an attractive alternative approach to immunotherapy. However, its efficacy remains insufficiently evaluated in preclinical studies. Here, we investigated HCC-associated self/tumor antigen, α-fetoprotein-based (AFP-based) vaccine immunization for treating AFP (+) HCC mouse models. We found that AFP immunization effectively induced AFP-specific CD8+ T cells in vivo. However, these CD8+ T cells expressed exhaustion markers, including PD1, LAG3, and Tim3. Furthermore, the AFP vaccine effectively prevented c-MYC/Mcl1 HCC initiation when administered before tumor formation, while it was ineffective against full-blown c-MYC/Mcl1 tumors. Similarly, anti-PD1 and anti-PD-L1 monotherapy showed no efficacy in this murine HCC model. In striking contrast, AFP immunization combined with anti-PD-L1 treatment triggered significant inhibition of HCC progression in most liver tumor nodules, while in combination with anti-PD1, it induced slower tumor progression. Mechanistically, we demonstrated that HCC-intrinsic PD-L1 expression was the primary target of anti-PD-L1 in this combination therapy. Notably, the combination therapy had a similar therapeutic effect in the cMet/β-catenin mouse HCC model. These findings suggest that combining the AFP vaccine and immune checkpoint inhibitors may be effective for AFP (+) HCC treatment.
Keywords: Hepatology; Immunology; Immunotherapy; Liver cancer; T cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

