Delayed adverse reactions in whole blood donors: Importance of active surveillance in identifying the missing gaps in the donor safety
- PMID: 37040224
- PMCID: PMC10284355
- DOI: 10.4103/ijmr.IJMR_1273_19
Delayed adverse reactions in whole blood donors: Importance of active surveillance in identifying the missing gaps in the donor safety
Abstract
Background & objectives: The information available regarding delayed adverse donor reactions (D-ADRs) is limited. Proactive follow up of donors for delayed reactions is not done routinely. This study was undertaken to analyze frequency and type of D-ADRs in whole blood donors as also the contributory factors.
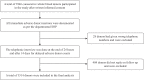
Methods: In this prospective observational study, all eligible whole blood donors were contacted telephonically twice (24 h and 2 wks after donation) and asked about general health and ADR specific questions. The International Society of Blood Transfusion standard guidelines were used to categorize ADRs.
Results: The ADR data of 3514 donors were analyzed in the study. D-ADRs were more common as compared to immediate delayed adverse donor reactions (I-ADRs) (13.7 vs. 2.9%, P<0.001). The most common D-ADRs were bruises (4.98%), fatigue or generalized weakness (4.24%) and sore arms (2.25%). D-ADRs were more common in first time donors as compared to the repeat blood donors (16.1 vs. 12.5%, P=0.002). Females were more prone to D-ADRs (17 vs. 13.6%). Localized D-ADRs were more frequent as compared to systemic D-ADRs (P<0.001). Repeat donors had a lower incidence of systemic D-ADRs (4.11% vs. 7.37%, P<0.001).
Interpretation & conclusions: D-ADRs were more common than I-ADRs with a different profile. First time, female and young donors were more prone to D-ADRs. These categories need special care at the time of blood donation. Active follow up of blood donors should be done from time to time to strengthen donor safety.
Keywords: Adverse donor reactions; blood donors; delayed reactions; donor safety; haematoma; vaso-vagal reactions; weakness.
Conflict of interest statement
Figures
References
-
- Directorate General of Health Services. Ministry of Health and Family Welfare, Government of India. Transfusion medicine technical manual. 2nd ed. India: DGHS, MoHFW, GoI; 2003.
-
- Desai PD, Navkudkar AA, Rajadhyaksha SB. On-site and off-site adverse donor reactions in voluntary whole blood donors: A study from a tertiary care oncology center. Glob J Transfus Med. 2019;4:28–32.
-
- Abhishekh B, Mayadevi S, Usha KC. Adverse reactions to blood donation. Innov J Med Health Sci. 2013;3:158–60.
-
- van Dongen A, Abraham C, Ruiter RA, Veldhuizen IJ. The influence of adverse reactions, subjective distress, and anxiety on retention of first-time blood donors. Transfusion. 2013;53:337–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials