Health and Disease: Akkermansia muciniphila, the Shining Star of the Gut Flora
- PMID: 37040299
- PMCID: PMC10079265
- DOI: 10.34133/research.0107
Health and Disease: Akkermansia muciniphila, the Shining Star of the Gut Flora
Abstract
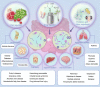
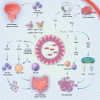
Akkermansia muciniphila (A. muciniphila) has drawn much attention as an important gut microbe strain in recent years. A. muciniphila can influence the occurrence and development of diseases of the endocrine, nervous, digestive, musculoskeletal, and respiratory systems and other diseases. It can also improve immunotherapy for some cancers. A. muciniphila is expected to become a new probiotic in addition to Lactobacillus and Bifidobacterium. An increase in A. muciniphila abundance through direct or indirect A. muciniphila supplementation may inhibit or even reverse disease progression. However, some contrary findings are found in type 2 diabetes mellitus and neurodegenerative diseases, where increased A. muciniphila abundance may aggravate the diseases. To enable a more comprehensive understanding of the role of A. muciniphila in diseases, we summarize the relevant information on A. muciniphila in different systemic diseases and introduce regulators of A. muciniphila abundance to promote the clinical transformation of A. muciniphila research.
Figures


References
-
- Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

