A mutagenesis study of autoantigen optimization for potential T1D vaccine design
- PMID: 37040399
- PMCID: PMC10120010
- DOI: 10.1073/pnas.2214430120
A mutagenesis study of autoantigen optimization for potential T1D vaccine design
Abstract
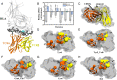
A previously reported autoreactive antigen, termed the X-idiotype, isolated from a unique cell population in Type 1 diabetes (T1D) patients, was found to stimulate their CD4+ T cells. This antigen was previously determined to bind more favorably than insulin and its mimic (insulin superagonist) to HLA-DQ8, supporting its strong role in CD4+ T cell activation. In this work, we probed HLA-X-idiotype-TCR binding and designed enhanced-reactive pHLA-TCR antigens using an in silico mutagenesis approach which we functionally validated by cell proliferation assays and flow cytometry. From a combination of single, double, and swap mutations, we identified antigen-binding sites p4 and p6 as potential mutation sites for HLA binding affinity enhancement. Site p6 is revealed to favor smaller but more hydrophobic residues than the native tyrosine, such as valine (Y6V) and isoleucine (Y6I), indicating a steric mechanism in binding affinity improvement. Meanwhile, site p4 methionine mutation to hydrophobic residues isoleucine (M4I) or leucine (M4L) modestly increases HLA binding affinity. Select p6 mutations to cysteine (Y6C) or isoleucine (Y6I) exhibit favorable TCR binding affinities, while a swap p5-p6 tyrosine-valine double mutant (V5Y_Y6V) and a p6-p7 glutamine-glutamine double mutant (Y6Q_Y7Q) exhibit enhanced HLA binding affinity but weakened TCR affinity. This work holds relevance to potential T1D antigen-based vaccine design and optimization.
Keywords: autoantigen design; free energy perturbation; molecular dynamics; mutagenesis; type 1 diabetes.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 2021YFF1200404/MOST | National Key Research and Development Program of China (NKPs)
- U1967217/National Natural Science Foundation of China (NSFC)
- 226-2022-00043/CSU | Fundamental Research Funds for Central Universities of the Central South University (Fundamental Research Funds for the Central Universities of the Central South University)
- SN-ZJU-SIAS-003/Starry Night Science Fund
- 2019-2022/W. M. Keck Foundation (WMKF)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

