ABL kinases regulate the stabilization of HIF-1α and MYC through CPSF1
- PMID: 37040401
- PMCID: PMC10120083
- DOI: 10.1073/pnas.2210418120
ABL kinases regulate the stabilization of HIF-1α and MYC through CPSF1
Abstract
The hypoxia-inducible factor 1-α (HIF-1α) enables cells to adapt and respond to hypoxia (Hx), and the activity of this transcription factor is regulated by several oncogenic signals and cellular stressors. While the pathways controlling normoxic degradation of HIF-1α are well understood, the mechanisms supporting the sustained stabilization and activity of HIF-1α under Hx are less clear. We report that ABL kinase activity protects HIF-1α from proteasomal degradation during Hx. Using a fluorescence-activated cell sorting (FACS)-based CRISPR/Cas9 screen, we identified HIF-1α as a substrate of the cleavage and polyadenylation specificity factor-1 (CPSF1), an E3-ligase which targets HIF-1α for degradation in the presence of an ABL kinase inhibitor in Hx. We show that ABL kinases phosphorylate and interact with CUL4A, a cullin ring ligase adaptor, and compete with CPSF1 for CUL4A binding, leading to increased HIF-1α protein levels. Further, we identified the MYC proto-oncogene protein as a second CPSF1 substrate and show that active ABL kinase protects MYC from CPSF1-mediated degradation. These studies uncover a role for CPSF1 in cancer pathobiology as an E3-ligase antagonizing the expression of the oncogenic transcription factors, HIF-1α and MYC.
Keywords: ABL kinases; CPSF1; E3-ligase; HIF-1α; MYC.
Conflict of interest statement
A.M.P. is a consultant and advisory board member for The Pew Charitable Trusts. All other authors declare that they have no competing interests.
Figures
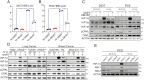




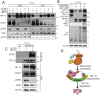



Similar articles
-
PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation.J Biol Chem. 2004 Apr 2;279(14):13506-13. doi: 10.1074/jbc.M310164200. Epub 2004 Jan 15. J Biol Chem. 2004. PMID: 14726529
-
Mechanisms of c-myc degradation by nickel compounds and hypoxia.PLoS One. 2009 Dec 31;4(12):e8531. doi: 10.1371/journal.pone.0008531. PLoS One. 2009. PMID: 20046830 Free PMC article.
-
Hypoxia induced E-cadherin involving regulators of Hippo pathway due to HIF-1α stabilization/nuclear translocation in bone metastasis from breast carcinoma.Exp Cell Res. 2015 Jan 15;330(2):287-299. doi: 10.1016/j.yexcr.2014.10.004. Epub 2014 Oct 19. Exp Cell Res. 2015. Retraction in: Exp Cell Res. 2020 Aug 15;393(2):112164. doi: 10.1016/j.yexcr.2020.112164. PMID: 25447306 Retracted.
-
RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization.Cell Cycle. 2007 Mar 15;6(6):656-9. doi: 10.4161/cc.6.6.3981. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361105 Review.
-
Regulation of the SIAH2-HIF-1 Axis by Protein Kinases and Its Implication in Cancer Therapy.Front Cell Dev Biol. 2021 Mar 25;9:646687. doi: 10.3389/fcell.2021.646687. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842469 Free PMC article. Review.
Cited by
-
Use of antibodies against Epstein-Barr virus nuclear antigen 1 for detection of cellular proteins with monomethylated arginine residues that are potentially involved in viral transformation.Arch Virol. 2024 Nov 8;169(12):241. doi: 10.1007/s00705-024-06172-7. Arch Virol. 2024. PMID: 39514105 Free PMC article.
-
Mutant TP53 promotes invasion of lung cancer cells by regulating desmoglein 3.J Cancer Res Clin Oncol. 2024 Jun 20;150(6):312. doi: 10.1007/s00432-024-05778-3. J Cancer Res Clin Oncol. 2024. PMID: 38900156 Free PMC article.
-
Mycobacterium tuberculosis-dependent monocyte expression quantitative trait loci, cytokine production, and TB pathogenesis.Front Immunol. 2024 Mar 7;15:1359178. doi: 10.3389/fimmu.2024.1359178. eCollection 2024. Front Immunol. 2024. PMID: 38515745 Free PMC article.
References
-
- Kaelin W. G. Jr., Ratcliffe P. J., Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008). - PubMed
-
- Kaelin W. G. Jr., The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 (2008). - PubMed
-
- Ivan M., et al. , HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292, 464–468 (2001). - PubMed
-
- Jaakkola P., et al. , Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

