B cell peripheral tolerance is promoted by cathepsin B protease
- PMID: 37040412
- PMCID: PMC10120085
- DOI: 10.1073/pnas.2300099120
B cell peripheral tolerance is promoted by cathepsin B protease
Abstract
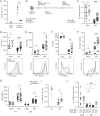
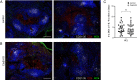
B cells that bind soluble autoantigens receive chronic signaling via the B cell receptor (signal-1) in the absence of strong costimulatory signals (signal-2), and this leads to their elimination in peripheral tissues. The factors determining the extent of soluble autoantigen-binding B cell elimination are not fully understood. Here we demonstrate that the elimination of B cells chronically exposed to signal-1 is promoted by cathepsin B (Ctsb). Using hen egg lysozyme-specific (HEL-specific) immunoglobulin transgenic (MD4) B cells and mice harboring circulating HEL, we found improved survival and increased proliferation of HEL-binding B cells in Ctsb-deficient mice. Bone marrow chimera experiments established that both hematopoietic and nonhematopoietic sources of Ctsb were sufficient to promote peripheral B cell deletion. The depletion of CD4+ T cells overcame the survival and growth advantage provided by Ctsb deficiency, as did blocking CD40L or removing CD40 from the chronically antigen-engaged B cells. Thus, we suggest that Ctsb acts extracellularly to reduce soluble autoantigen-binding B cell survival and that its actions restrain CD40L-dependent pro-survival effects. These findings identify a role for cell-extrinsic protease activity in establishing a peripheral self-tolerance checkpoint.
Keywords: B lymphocyte; CD40; cathepsin; self-tolerance; signal-1.
Conflict of interest statement
J.G.C. is on the scientific advisory board of BeBio Pharma and MiroBio Ltd.
Figures


Similar articles
-
Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival.Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10717-22. doi: 10.1073/pnas.0601539103. Epub 2006 Jun 30. Proc Natl Acad Sci U S A. 2006. PMID: 16815973 Free PMC article.
-
Follicular exclusion and rapid elimination of hen egg lysozyme autoantigen-binding B cells are dependent on competitor B cells, but not on T cells.J Immunol. 1999 Jan 1;162(1):284-91. J Immunol. 1999. PMID: 9886397
-
CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells.Nature. 1995 Jul 13;376(6536):181-4. doi: 10.1038/376181a0. Nature. 1995. PMID: 7603571
-
Human rheumatoid factor production is dependent on CD40 signaling and autoantigen.J Immunol. 1999 Sep 15;163(6):3116-22. J Immunol. 1999. PMID: 10477577
-
The role of CD40 ligand in costimulation and T-cell activation.Immunol Rev. 1996 Oct;153:85-106. doi: 10.1111/j.1600-065x.1996.tb00921.x. Immunol Rev. 1996. PMID: 9010720 Review.
Cited by
-
Molecular basis for potent B cell responses to antigen displayed on particles of viral size.Nat Immunol. 2023 Oct;24(10):1762-1777. doi: 10.1038/s41590-023-01597-9. Epub 2023 Aug 31. Nat Immunol. 2023. PMID: 37653247 Free PMC article.
-
Platinum(IV) anticancer therapies and cathepsin B: innovative strategies for overcoming resistance in glioblastoma cells.Front Cell Dev Biol. 2025 Jun 4;13:1506206. doi: 10.3389/fcell.2025.1506206. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40535571 Free PMC article.
-
Cathepsin B in urological tumors: unraveling its role and therapeutic potential.Discov Oncol. 2025 May 9;16(1):707. doi: 10.1007/s12672-025-02552-w. Discov Oncol. 2025. PMID: 40343561 Free PMC article. Review.
-
Cathepsin B in cardiovascular disease: Underlying mechanisms and therapeutic strategies.J Cell Mol Med. 2024 Sep;28(17):e70064. doi: 10.1111/jcmm.70064. J Cell Mol Med. 2024. PMID: 39248527 Free PMC article. Review.
References
-
- Goodnow C. C., Sprent J., Fazekas de St Groth B., Vinuesa C. G., Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435, 590–597 (2005). - PubMed
-
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A., Induction of self-tolerance in mature peripheral B lymphocytes. Nature 342, 385–391 (1989). - PubMed
-
- Cyster J. G., Hartley S. B., Goodnow C. C., Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature 371, 389–395 (1994). - PubMed
-
- Cyster J. G., Goodnow C. C., Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity 3, 691–701 (1995). - PubMed
-
- Lesley R., et al. , Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20, 441–453 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

