HSP90 inhibition in the mouse spinal cord enhances opioid signaling by suppressing an AMPK-mediated negative feedback loop
- PMID: 37040443
- PMCID: PMC11010773
- DOI: 10.1126/scisignal.ade2438
HSP90 inhibition in the mouse spinal cord enhances opioid signaling by suppressing an AMPK-mediated negative feedback loop
Abstract
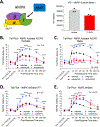
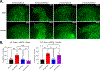
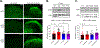
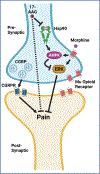
Opioids and other agonists of the μ-opioid receptor are effective at managing acute pain, but their chronic use can lead to tolerance that limits their efficacy. We previously reported that inhibiting the chaperone protein HSP90 in the spinal cords of mice promotes the antinociceptive effects of opioids in a manner that involved increased activation of the kinase ERK. Here, we found that the underlying mechanism involves the relief of a negative feedback loop mediated by the kinase AMPK. Intrathecal treatment of male and female mice with the HSP90 inhibitor 17-AAG decreased the abundance of the β1 subunit of AMPK in the spinal cord. The antinociceptive effects of 17-AAG with morphine were suppressed by intrathecal administration of AMPK activators and enhanced by an AMPK inhibitor. Opioid treatment increased the abundance of phosphorylated AMPK in the dorsal horn of the spinal cord, where it colocalized with a neuronal marker and the neuropeptide CGRP. Knocking down AMPK in CGRP-positive neurons enhanced the antinociceptive effects of morphine and demonstrated that AMPK mediated the signal transduction between HSP90 inhibition and ERK activation. These data suggest that AMPK mediates an opioid-induced negative feedback loop in CGRP neurons of the spinal cord and that this loop can be disabled by HSP90 inhibition to enhance the efficacy of opioids.
Conflict of interest statement
Figures
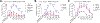


Similar articles
-
Inhibition of Hsp90 in the spinal cord enhances the antinociceptive effects of morphine by activating an ERK-RSK pathway.Sci Signal. 2020 May 5;13(630):eaaz1854. doi: 10.1126/scisignal.aaz1854. Sci Signal. 2020. PMID: 32371496 Free PMC article.
-
Inhibiting spinal cord-specific hsp90 isoforms reveals a novel strategy to improve the therapeutic index of opioid treatment.Sci Rep. 2024 Jun 26;14(1):14715. doi: 10.1038/s41598-024-65637-6. Sci Rep. 2024. PMID: 38926482 Free PMC article.
-
Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation.J Neuroinflammation. 2016 Nov 17;13(1):294. doi: 10.1186/s12974-016-0754-9. J Neuroinflammation. 2016. PMID: 27855689 Free PMC article.
-
Mitochondrial-Derived Peptide MOTS-c Ameliorates Spared Nerve Injury-Induced Neuropathic Pain in Mice by Inhibiting Microglia Activation and Neuronal Oxidative Damage in the Spinal Cord via the AMPK Pathway.ACS Chem Neurosci. 2023 Jun 21;14(12):2362-2374. doi: 10.1021/acschemneuro.3c00140. Epub 2023 Jun 7. ACS Chem Neurosci. 2023. PMID: 37285113
-
Opioidergic control of the spinal release of neuropeptides. Possible significance for the analgesic effects of opioids.Fundam Clin Pharmacol. 1994;8(4):307-21. doi: 10.1111/j.1472-8206.1994.tb00809.x. Fundam Clin Pharmacol. 1994. PMID: 7851837 Review.
Cited by
-
Select terpenes from Cannabis sativa are antinociceptive in mouse models of post-operative pain and fibromyalgia via adenosine A2a receptors.Pharmacol Rep. 2025 Feb;77(1):172-181. doi: 10.1007/s43440-024-00687-1. Epub 2024 Dec 12. Pharmacol Rep. 2025. PMID: 39663308
-
Genetic editing of primary human dorsal root ganglion neurons using CRISPR-Cas9.Sci Rep. 2025 Apr 1;15(1):11116. doi: 10.1038/s41598-025-91153-2. Sci Rep. 2025. PMID: 40169710 Free PMC article.
-
Microglia in morphine tolerance: cellular and molecular mechanisms and therapeutic potential.Front Pharmacol. 2024 Nov 27;15:1499799. doi: 10.3389/fphar.2024.1499799. eCollection 2024. Front Pharmacol. 2024. PMID: 39669194 Free PMC article. Review.
-
Increased TSPO alleviates neuropathic pain by preventing pyroptosis via the AMPK-PGC-1α pathway.J Headache Pain. 2025 Jan 27;26(1):16. doi: 10.1186/s10194-025-01953-0. J Headache Pain. 2025. PMID: 39871133 Free PMC article.
-
The Hsp90β Isoform: An Attractive Target for Drug Development.Med Res Rev. 2025 Sep;45(5):1452-1465. doi: 10.1002/med.22114. Epub 2025 Apr 28. Med Res Rev. 2025. PMID: 40293270 Free PMC article. Review.
References
-
- Berthelot JM, Darrieutort-Lafitte C, Le Goff B, Maugars Y, Strong opioids for noncancer pain due to musculoskeletal diseases: Not more effective than acetaminophen or NSAIDs. Joint Bone Spine 82, 397–401 (2015). - PubMed
-
- Zöllner C, Stein C, Opioids. analgesia, 31–63 (2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

