Oncolytic virotherapy: basic principles, recent advances and future directions
- PMID: 37041165
- PMCID: PMC10090134
- DOI: 10.1038/s41392-023-01407-6
Oncolytic virotherapy: basic principles, recent advances and future directions
Abstract
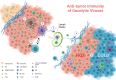
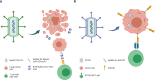
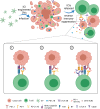
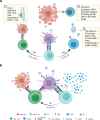
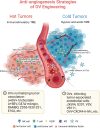
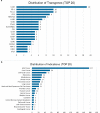
Oncolytic viruses (OVs) have attracted growing awareness in the twenty-first century, as they are generally considered to have direct oncolysis and cancer immune effects. With the progress in genetic engineering technology, OVs have been adopted as versatile platforms for developing novel antitumor strategies, used alone or in combination with other therapies. Recent studies have yielded eye-catching results that delineate the promising clinical outcomes that OVs would bring about in the future. In this review, we summarized the basic principles of OVs in terms of their classifications, as well as the recent advances in OV-modification strategies based on their characteristics, biofunctions, and cancer hallmarks. Candidate OVs are expected to be designed as "qualified soldiers" first by improving target fidelity and safety, and then equipped with "cold weapons" for a proper cytocidal effect, "hot weapons" capable of activating cancer immunotherapy, or "auxiliary weapons" by harnessing tactics such as anti-angiogenesis, reversed metabolic reprogramming and decomposing extracellular matrix around tumors. Combinations with other cancer therapeutic agents have also been elaborated to show encouraging antitumor effects. Robust results from clinical trials using OV as a treatment congruously suggested its significance in future application directions and challenges in developing OVs as novel weapons for tactical decisions in cancer treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

