Characterisation of NPFF-expressing neurons in the superficial dorsal horn of the mouse spinal cord
- PMID: 37041197
- PMCID: PMC10090074
- DOI: 10.1038/s41598-023-32720-3
Characterisation of NPFF-expressing neurons in the superficial dorsal horn of the mouse spinal cord
Abstract
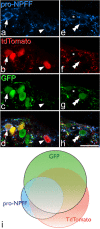
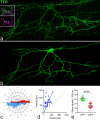
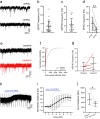
Excitatory interneurons in the superficial dorsal horn (SDH) are heterogeneous, and include a class known as vertical cells, which convey information to lamina I projection neurons. We recently used pro-NPFF antibody to reveal a discrete population of excitatory interneurons that express neuropeptide FF (NPFF). Here, we generated a new mouse line (NPFFCre) in which Cre is knocked into the Npff locus, and used Cre-dependent viruses and reporter mice to characterise NPFF cell properties. Both viral and reporter strategies labelled many cells in the SDH, and captured most pro-NPFF-immunoreactive neurons (75-80%). However, the majority of labelled cells lacked pro-NPFF, and we found considerable overlap with a population of neurons that express the gastrin-releasing peptide receptor (GRPR). Morphological reconstruction revealed that most pro-NPFF-containing neurons were vertical cells, but these differed from GRPR neurons (which are also vertical cells) in having a far higher dendritic spine density. Electrophysiological recording showed that NPFF cells also differed from GRPR cells in having a higher frequency of miniature EPSCs, being more electrically excitable and responding to a NPY Y1 receptor agonist. Together, these findings indicate that there are at least two distinct classes of vertical cells, which may have differing roles in somatosensory processing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

