Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia
- PMID: 37041198
- PMCID: PMC10244159
- DOI: 10.1038/s41375-023-01884-2
Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia
Abstract
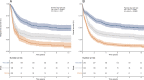
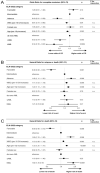
The revised 2022 European LeukemiaNet (ELN) AML risk stratification system requires validation in large, homogeneously treated cohorts. We studied 1118 newly diagnosed AML patients (median age, 58 years; range, 18-86 years) who received cytarabine-based induction chemotherapy between 1999 and 2012 and compared ELN-2022 to the previous ELN-2017 risk classification. Key findings were validated in a cohort of 1160 mostly younger patients. ELN-2022 reclassified 15% of patients, 3% into more favorable, and 12% into more adverse risk groups. This was mainly driven by patients reclassified from intermediate- to adverse-risk based on additional myelodysplasia-related mutations being included as adverse-risk markers. These patients (n = 79) had significantly better outcomes than patients with other adverse-risk genotypes (5-year OS, 26% vs. 12%) and resembled the remaining intermediate-risk group. Overall, time-dependent ROC curves and Harrel's C-index controlling for age, sex, and AML type (de novo vs. sAML/tAML) show slightly worse prognostic discrimination of ELN-2022 compared to ELN-2017 for OS. Further refinement of ELN-2022 without including additional genetic markers is possible, in particular by recognizing TP53-mutated patients with complex karyotypes as "very adverse". In summary, the ELN-2022 risk classification identifies a larger group of adverse-risk patients at the cost of slightly reduced prognostic accuracy compared to ELN-2017.
© 2023. The Author(s).
Conflict of interest statement
HG is a current employee of Roche Pharma AG, Grenzach-Wyhlen, Germany. The other authors declare no competing interests.
Figures




References
-
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

