Regulating GSDMB pore formation: to ignite or inhibit?
- PMID: 37041290
- PMCID: PMC10244342
- DOI: 10.1038/s41418-023-01163-8
Regulating GSDMB pore formation: to ignite or inhibit?
Abstract
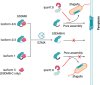
In the recent Nature, Wang et al. and Zhong et al. present the cryo-EM structures of Gasdermin B (GSDMB) pore and strucures of GSDMB in complex with a Shigella effector, IpaH7.8. The structures shed light on the structural mechanisms that govern GSDMB-mediated pyroptosis, a process that is regulated by pathogenic bacteria and alternative splicing.
Conflict of interest statement
The author declares no competing interests.
Figures
Similar articles
-
New Insights Relating Gasdermin B to the Onset of Childhood Asthma.Am J Respir Cell Mol Biol. 2022 Oct;67(4):430-437. doi: 10.1165/rcmb.2022-0043PS. Am J Respir Cell Mol Biol. 2022. PMID: 35580164 Free PMC article. Review.
-
Structural mechanisms for regulation of GSDMB pore-forming activity.Nature. 2023 Apr;616(7957):598-605. doi: 10.1038/s41586-023-05872-5. Epub 2023 Mar 29. Nature. 2023. PMID: 36991125
-
Structural basis for GSDMB pore formation and its targeting by IpaH7.8.Nature. 2023 Apr;616(7957):590-597. doi: 10.1038/s41586-023-05832-z. Epub 2023 Mar 29. Nature. 2023. PMID: 36991122 Free PMC article.
-
ORMDL3/GSDMB genotype is associated with distinct phenotypes of adult asthma.Allergol Int. 2021 Oct;70(4):495-497. doi: 10.1016/j.alit.2021.04.004. Epub 2021 Apr 30. Allergol Int. 2021. PMID: 33941434 No abstract available.
-
Chromosome 17q21 Genes ORMDL3 and GSDMB in Asthma and Immune Diseases.Adv Immunol. 2017;135:1-52. doi: 10.1016/bs.ai.2017.06.001. Epub 2017 Jul 19. Adv Immunol. 2017. PMID: 28826527 Review.
Cited by
-
Functional Materials Targeted Regulation of Gasdermins: From Fundamentals to Functionalities and Applications.Adv Sci (Weinh). 2025 Apr;12(16):e2500873. doi: 10.1002/advs.202500873. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40273335 Free PMC article. Review.
-
Modeling of horizontal pleiotropy identifies possible causal gene expression in systemic lupus erythematosus.Front Lupus. 2023;1:1234578. doi: 10.3389/flupu.2023.1234578. Epub 2023 Oct 3. Front Lupus. 2023. PMID: 37799268 Free PMC article.
References
-
- Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020; 368:eaaz7548. - PubMed
-
- Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. 2018;3. 10.1126/sciimmunol.aar6689. - PubMed

