An assessment of the strategy and status of COVID-19 vaccination in India
- PMID: 37041424
- PMCID: PMC10089693
- DOI: 10.1007/s12026-023-09373-5
An assessment of the strategy and status of COVID-19 vaccination in India
Abstract
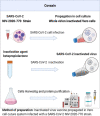
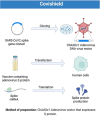
The COVID-19 disease continues to cause devastation for almost 3 years of its identification. India is one of the leading countries to set clinical trials, production, and administration of COVID-19 vaccination. Recent COVID-19 vaccine tracker record suggests that 12 vaccines are approved in India, including protein subunit, RNA/DNA, non-replicating viral vector, and inactivated vaccine. Along with that 16 more vaccines are undergoing clinical trials to counter COVID-19. The availability of different vaccines gives alternate and broad perspectives to fight against viral immune resistance and, thus, viruses escaping the immune system by mutations. Using the recently published literature on the Indian vaccine and clinical trial sites, we have reviewed the development, clinical evaluation, and registration of vaccines trial used in India against COVID-19. Moreover, we have also summarized the status of all approved vaccines in India, their associated registered clinical trials, manufacturing, efficacy, and their related safety and immunogenicity profile.
Keywords: Covaxin; Covishield; SARS-CoV-2; Sputnik; Vaccination.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

