Bionate® nucleus disc replacement: bench testing comparing two different designs
- PMID: 37041425
- PMCID: PMC10090247
- DOI: 10.1186/s10195-023-00692-9
Bionate® nucleus disc replacement: bench testing comparing two different designs
Abstract
Background: Intervertebral disc nucleus degeneration initiates a degenerative cascade and can induce chronic low back pain. Nucleus replacement aims to replace the nucleus while the annulus is still intact. Over time, several designs have been introduced, but the definitive solution continues to be elusive. Therefore, we aimed to create a new nucleus replacement that replicates intact intervertebral disc biomechanics, and thus has the potential for clinical applications.
Materials and methods: Two implants with an outer ring and one (D2) with an additional midline strut were compared. Static and fatigue tests were performed with an INSTRON 8874 following the American Society for Testing and Materials F2267-04, F2346-05, 2077-03, D2990-01, and WK4863. Implant stiffness was analyzed at 0-300 N, 500-2000 N, and 2000-6000 N and implant compression at 300 N, 1000 N, 2000 N, and 6000 N. Wear tests were performed following ISO 18192-1:2008 and 18192-2:2010. GNU Octave software was used to calculate movement angles and parameters. The statistical analysis package R was used with the Deducer user interface. Statistically significant differences between the two designs were analyzed with ANOVA, followed by a post hoc analysis.
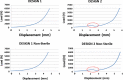
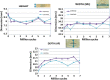
Results: D1 had better behavior in unconfined compression tests, while D2 showed a "jump." D2 deformed 1 mm more than D1. Sterilized implants were more rigid and deformed less. Both designs showed similar behavior under confined compression and when adding shear. A silicone annulus minimized differences between the designs. Wear under compression fatigue was negligible for D1 but permanent for D2. D1 suffered permanent height deformation but kept its width. D2 suffered less height loss than D1 but underwent a permanent width deformation. Both designs showed excellent responses to compression fatigue with no breaks, cracks, or delamination. At 10 million cycles, D2 showed 3-times higher wear than D1. D1 had better and more homogeneous behavior, and its wear was relatively low. It showed good mechanical endurance under dynamic loading conditions, with excellent response to axial compression fatigue loading without functional failure after long-term testing.
Conclusion: D1 performed better than D2. Further studies in cadaveric specimens, and eventually in a clinical setting, are recommended. Level of evidence 2c.
Keywords: Degenerative disc disease; Finite element analysis; Motion preservation; Nucleus disc replacement; Polycarbonate urethane.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests relevant to this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interests (such as honoraria; educational grants; participation in speaker’s bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interests (such as personal or professional relationships, affiliations, knowledge or belief(s) in the subject matter or materials discussed in this manuscript).
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

