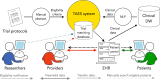
Piloting an automated clinical trial eligibility surveillance and provider alert system based on artificial intelligence and standard data models
- PMID: 37041475
- PMCID: PMC10088225
- DOI: 10.1186/s12874-023-01916-6
Piloting an automated clinical trial eligibility surveillance and provider alert system based on artificial intelligence and standard data models
Abstract
Background: To advance new therapies into clinical care, clinical trials must recruit enough participants. Yet, many trials fail to do so, leading to delays, early trial termination, and wasted resources. Under-enrolling trials make it impossible to draw conclusions about the efficacy of new therapies. An oft-cited reason for insufficient enrollment is lack of study team and provider awareness about patient eligibility. Automating clinical trial eligibility surveillance and study team and provider notification could offer a solution.
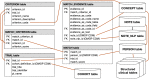
Methods: To address this need for an automated solution, we conducted an observational pilot study of our TAES (TriAl Eligibility Surveillance) system. We tested the hypothesis that an automated system based on natural language processing and machine learning algorithms could detect patients eligible for specific clinical trials by linking the information extracted from trial descriptions to the corresponding clinical information in the electronic health record (EHR). To evaluate the TAES information extraction and matching prototype (i.e., TAES prototype), we selected five open cardiovascular and cancer trials at the Medical University of South Carolina and created a new reference standard of 21,974 clinical text notes from a random selection of 400 patients (including at least 100 enrolled in the selected trials), with a small subset of 20 notes annotated in detail. We also developed a simple web interface for a new database that stores all trial eligibility criteria, corresponding clinical information, and trial-patient match characteristics using the Observational Medical Outcomes Partnership (OMOP) common data model. Finally, we investigated options for integrating an automated clinical trial eligibility system into the EHR and for notifying health care providers promptly of potential patient eligibility without interrupting their clinical workflow.
Results: Although the rapidly implemented TAES prototype achieved only moderate accuracy (recall up to 0.778; precision up to 1.000), it enabled us to assess options for integrating an automated system successfully into the clinical workflow at a healthcare system.
Conclusions: Once optimized, the TAES system could exponentially enhance identification of patients potentially eligible for clinical trials, while simultaneously decreasing the burden on research teams of manual EHR review. Through timely notifications, it could also raise physician awareness of patient eligibility for clinical trials.
Keywords: Clinical trial enrollment/recruitment; Data science [L01.305]; Medical informatics [L01.313.500]; Natural language processing (NLP) [L01.224.050.375.580].
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Automatic trial eligibility surveillance based on unstructured clinical data.Int J Med Inform. 2019 Sep;129:13-19. doi: 10.1016/j.ijmedinf.2019.05.018. Epub 2019 May 23. Int J Med Inform. 2019. PMID: 31445247 Free PMC article.
-
Automated clinical trial eligibility prescreening: increasing the efficiency of patient identification for clinical trials in the emergency department.J Am Med Inform Assoc. 2015 Jan;22(1):166-78. doi: 10.1136/amiajnl-2014-002887. Epub 2014 Jul 16. J Am Med Inform Assoc. 2015. PMID: 25030032 Free PMC article.
-
Increasing the efficiency of trial-patient matching: automated clinical trial eligibility pre-screening for pediatric oncology patients.BMC Med Inform Decis Mak. 2015 Apr 14;15:28. doi: 10.1186/s12911-015-0149-3. BMC Med Inform Decis Mak. 2015. PMID: 25881112 Free PMC article.
-
Machine learning and natural language processing in clinical trial eligibility criteria parsing: a scoping review.Drug Discov Today. 2024 Oct;29(10):104139. doi: 10.1016/j.drudis.2024.104139. Epub 2024 Aug 19. Drug Discov Today. 2024. PMID: 39154773
-
Large language models for automating clinical trial matching.Curr Opin Urol. 2025 May 1;35(3):250-258. doi: 10.1097/MOU.0000000000001281. Epub 2025 Mar 20. Curr Opin Urol. 2025. PMID: 40114652 Review.
Cited by
-
Clinical Research Informatics: a Decade-in-Review.Yearb Med Inform. 2024 Aug;33(1):127-142. doi: 10.1055/s-0044-1800732. Epub 2025 Apr 8. Yearb Med Inform. 2024. PMID: 40199298 Free PMC article. Review.
-
Advances in nanotechnology-enabled adjuvants for peptide-based cancer vaccines.Nano Res. 2025 Jul;18(7):94907534. doi: 10.26599/nr.2025.94907534. Epub 2025 May 22. Nano Res. 2025. PMID: 40837119 Free PMC article.
-
Artificial intelligence for optimizing recruitment and retention in clinical trials: a scoping review.J Am Med Inform Assoc. 2024 Nov 1;31(11):2749-2759. doi: 10.1093/jamia/ocae243. J Am Med Inform Assoc. 2024. PMID: 39259922 Free PMC article.
-
Artificial intelligence-enhanced patient evaluation: bridging art and science.Eur Heart J. 2024 Sep 14;45(35):3204-3218. doi: 10.1093/eurheartj/ehae415. Eur Heart J. 2024. PMID: 38976371 Free PMC article. Review.
-
Development and implementation of cancer clinical trial patient screening using an electronic medical record-integrated trial matching system.BMJ Health Care Inform. 2025 Jul 16;32(1):e101295. doi: 10.1136/bmjhci-2024-101295. BMJ Health Care Inform. 2025. PMID: 40670040 Free PMC article.
References
-
- Sung NS, Crowley WF, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–1287. doi: 10.1001/jama.289.10.1278. - DOI - PubMed
-
- Dilts DM, Sandler AB. Activating & Opening Oncology Clinical Trials: Process & Timing Analysis. NCI presentation. 2008. Available at: https://deainfo.nci.nih.gov/advisory/bsa/archive/bsa0308/presentations/M....
-
- Lara PN, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, Wun T, Welborn J, Meyers FJ, Christensen S, O'Donnell R, Richman C, Scudder SA, Tuscano J, Gandara DR, Lam KS. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. doi: 10.1200/JCO.2001.19.6.1728. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources