A nomogram model based on pre-treatment and post-treatment MR imaging radiomics signatures: application to predict progression-free survival for nasopharyngeal carcinoma
- PMID: 37041545
- PMCID: PMC10088158
- DOI: 10.1186/s13014-023-02257-w
A nomogram model based on pre-treatment and post-treatment MR imaging radiomics signatures: application to predict progression-free survival for nasopharyngeal carcinoma
Abstract
Background: To establish a novel model using radiomics analysis of pre-treatment and post-treatment magnetic resonance (MR) images for prediction of progression-free survival in the patients with stage II-IVA nasopharyngeal carcinoma (NPC) in South China.
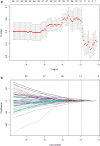
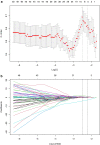
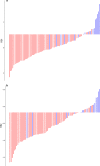
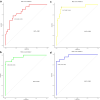
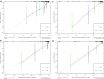
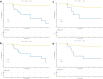
Methods: One hundred and twenty NPC patients who underwent chemoradiotherapy were enrolled (80 in the training cohort and 40 in the validation cohort). Acquiring data and screening features were performed successively. Totally 1133 radiomics features were extracted from the T2-weight images before and after treatment. Least absolute shrinkage and selection operator regression, recursive feature elimination algorithm, random forest, and minimum-redundancy maximum-relevancy (mRMR) method were used for feature selection. Nomogram discrimination and calibration were evaluated. Harrell's concordance index (C-index) and receiver operating characteristic (ROC) analyses were applied to appraise the prognostic performance of nomograms. Survival curves were plotted using Kaplan-Meier method.
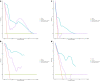
Results: Integrating independent clinical predictors with pre-treatment and post-treatment radiomics signatures which were calculated in conformity with radiomics features, we established a clinical-and-radiomics nomogram by multivariable Cox regression. Nomogram consisting of 14 pre-treatment and 7 post-treatment selected features has been proved to yield a reliable predictive performance in both training and validation groups. The C-index of clinical-and-radiomics nomogram was 0.953 (all P < 0.05), which was higher than that of clinical (0.861) or radiomics nomograms alone (based on pre-treatment statistics: 0.942; based on post-treatment statistics: 0.944). Moreover, we received Rad-score of pre-treatment named RS1 and post-treatment named RS2 and all were used as independent predictors to divide patients into high-risk and low-risk groups. Kaplan-Meier analysis showed that lower RS1 (less than cutoff value, - 1.488) and RS2 (less than cutoff value, - 0.180) were easier to avoid disease progression (all P < 0.01). It showed clinical benefit with decision curve analysis.
Conclusions: MR-based radiomics measured the burden on primary tumor before treatment and the tumor regression after chemoradiotherapy, and was used to build a model to predict progression-free survival (PFS) in the stage II-IVA NPC patients. It can also help to distinguish high-risk patients from low-risk patients, thus guiding personalized treatment decisions effectively.
Keywords: Magnetic resonance imaging; Nasopharyngeal carcinoma; Nomogram; Prognosis; Progression-free survival; Radiomics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, Deng X, Huang S, Lin C, Lu T. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi: 10.1016/j.radonc.2013.10.020. - DOI - PubMed
-
- Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27(22):3684–3690. doi: 10.1200/JCO.2008.19.9109. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

