A model for crowdsourcing high-impact research questions for Castleman disease and other rare diseases
- PMID: 37041585
- PMCID: PMC10091676
- DOI: 10.1186/s13023-023-02678-6
A model for crowdsourcing high-impact research questions for Castleman disease and other rare diseases
Abstract
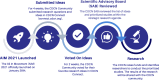
Background: There are approximately 10,000 rare diseases that affect around 30,000,000 individuals in the U.S.A., most of which do not have an FDA-approved treatment. This fact highlights the failure of traditional research approaches to overcome the unique challenges of developing rare disease treatments. The Castleman Disease Collaborative Network was founded in 2012 to advance research and treatments for Castleman disease, a rare and deadly disease that involves the immune system attacking the body's vital organs for an unknown cause. It has spearheaded a novel strategy for advancing biomedical research, the Collaborative Network Approach. This approach consists of eight steps, one of which is to identify and prioritize high-impact research questions through crowdsourcing ideas from the entire community of stakeholders: patients, loved ones, physicians, and researchers. Rather than hoping that the right researcher will apply for the right research project at the right time, crowdsourcing high-priority research projects into a research strategy ensures that the most high-impact, patient-centered studies are prioritized. The Castleman Disease Collaborative Network launched an initiative in 2021 to systematically generate this list of community-directed studies to focus Castleman disease research efforts.
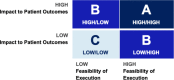
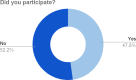
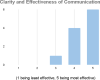
Results: The Castleman Disease Collaborative Network was able to successfully create a patient-centered research agenda through engaging the entire community of stakeholders. The community contributed important questions about Castleman disease, which were prioritized and reviewed by our Scientific Advisory Board, and the result was a finalized list of studies that address these prioritized questions. We were also able to generate a best practices list which can serve as a model that can be utilized for other rare diseases.
Conclusion: Creating a patient-centered research agenda through crowdsourcing research ideas from the community is one of the most important ways that the Castleman Disease Collaborative Network operationalizes its commitment to keeping patients at the center of research and we hope that by sharing these insights we can assist other rare disease organizations to pursue a patient-centric approach.
Keywords: Castleman disease; Collaborative network; Crowdsourcing; Patient-centered research agenda; Rare disease.
© 2023. The Author(s).
Conflict of interest statement
Not applicable.
Figures






References
-
- Orphan Drug Act [Internet]. 96 STAT. 2049, 97–414 Jan 4, 1983. Available from: https://www.fda.gov/media/99546/download
-
- Zuccato M, Shilling D, Fajgenbaum DC. The Collaborative Network Approach: a model for advancing patient-centric research for Castleman disease and other rare diseases. MacKenzie A, Groft S, Justice M, van Karnebeek C, editors. Emerging Topics in Life Sciences. 2019 Mar 29;3(1):97–105. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

