A qualitative study on the feasibility and acceptability of institutionalizing health technology assessment in Malawi
- PMID: 37041590
- PMCID: PMC10088659
- DOI: 10.1186/s12913-023-09276-z
A qualitative study on the feasibility and acceptability of institutionalizing health technology assessment in Malawi
Abstract
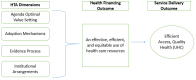
Objective: The objective of this study was to assess the feasibility and acceptability of institutionalizing Health Technology Assessment (HTA) in Malawi.
Methods: This study employed a document review and qualitative research methods, to understand the status of HTA in Malawi. This was complemented by a review of the status and nature of HTA institutionalization in selected countries.Qualitative research employed a Focus Group Discussion (FGD ) with 7 participants, and Key Informant Interviews (KIIs) with12 informants selected based on their knowledge and expertise in policy processes related to HTA in Malawi.Data extracted from the literature was organized in Microsoft Excel, categorized according to thematic areas and analyzed using a literature review framework. Qualitative data from KIIs and the FGD was analyzed using a thematic content analysis approach.
Results: Some HTA processes exist and are executed through three structures namely: Ministry of Health Senior Management Team, Technical Working Groups, and Pharmacy and Medicines Regulatory Authority (PMRA) with varyingdegrees of effectiveness.The main limitations of current HTA mechanisms include limited evidence use, lack of a standardized framework for technology adoption, donor pressure, lack of resources for the HTA process and technology acquisition, laws and practices that undermine cost-effectiveness considerations. KII and FGD results showed overwhelming demand for strengthening HTA in Malawi, with a stronger preference for strengthening coordination and capacity of existing entities and structures.
Conclusion: The study has shown that HTA institutionalization is acceptable and feasible in Malawi. However, the current committee based processes are suboptimal to improve efficiency due to lack of a structured framework. A structured HTA framework has the potential to improve processes in pharmaceuticals and medical technologies decision-making.In the short to medium term, HTA capacity building should focus on generating demand and increasing capacity in cost-effectiveness assessments. Country-specific assessments should precede HTA institutionalization as well as recommendations for new technology adoptions.
Keywords: Decision making; Health technology; Health technology assessment; Institutionalization; Malawi; Sub-Saharan Africa.
© 2023. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- Tantivess S, Chalkidou K, Tritasavit N, Teerawattananon Y. Health Technology Assessment capacity development in low- and middle-income countries: Experiences from the international units of HITAP and NICE [Internet]. F1000Research; 2017 [cited 2022 Oct 11]. Available from: https://f1000research.com/articles/6-2119 - PMC - PubMed
-
- Prinja S, Jyani G, Gupta N, Rajsekar K. Adapting health technology assessment for drugs, medical devices, and health programs: methodological considerations from the indian experience. Expert Rev Pharmacoecon Outcomes Res. 2021 Oct;21(5):859–68. - PubMed
-
- O’Rourke B, Oortwijn W, Schuller T, International Joint Task Group. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. 2020 Jun;36(3):187–90. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources