A Case of Vancomycin-Induced Drug Reaction with Eosinophilia, Systemic Symptoms and Multiorgan Involvement Proven Using Lymphocyte Transformation Test
- PMID: 37041708
- PMCID: PMC10112376
- DOI: 10.5021/ad.20.341
A Case of Vancomycin-Induced Drug Reaction with Eosinophilia, Systemic Symptoms and Multiorgan Involvement Proven Using Lymphocyte Transformation Test
Abstract
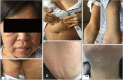
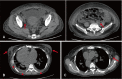
Drug-induced hypersensitivity syndrome (DiHS), also referred to as drug reaction with eosinophilia and systemic symptoms (DRESS), is a rare but potentially life-threatening condition induced by drug hypersensitivity that leads to significant morbidity and mortality and often occurs in patients undergoing combination antibiotic therapy. Due to a recent increase in the incidence of methicillin-resistant Staphylococcus aureus infections, the occurrence of vancomycin-induced DiHS/DRESS has increased rapidly. However, because of insufficient pharmacogenetic data on vancomycin-induced drug eruptions in Asians coupled with the risk of re-eliciting the symptoms by provocation tests, confirmation of the culprit drug in vancomycin-induced DiHS/DRESS is often challenging. Here, we report a case of vancomycin-induced DiHS/DRESS, where the causal relationship was confirmed using a lymphocyte transformation test (LTT). A 51-year-old woman was treated with combination antibiotics, including vancomycin, for infective pericarditis. The patient subsequently developed fever, facial edema, generalized rash followed by multiple internal organ involvement, including the kidney, lung, liver, and heart. Thus, based on the International Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria, the case was diagnosed as 'definite' DiHS/DRESS, although the culprit drug was obscured by combination antibiotic therapy. The LTT confirmed that vancomycin, but not other glycopeptide antibiotics, specifically induced T-cell proliferation in this case. Collectively, our case suggests that clinicians can utilize LTT to identify the causative medication of DiHS/DRESS when the clinical information is limited to defining the culprit drug.
Keywords: Drug eruptions; Drug hypersensitivity; Drug hypersensitivity syndrome; Lymphocyte activation; Vancomycin.
Copyright © The Korean Dermatological Association and The Korean Society for Investigative Dermatology.
Conflict of interest statement
The authors have nothing to disclose.
Figures

Similar articles
-
Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis.J Allergy Clin Immunol Pract. 2022 May;10(5):1155-1167.e5. doi: 10.1016/j.jaip.2022.02.004. Epub 2022 Feb 15. J Allergy Clin Immunol Pract. 2022. PMID: 35176506 Free PMC article.
-
RegiSCAR DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms) Validation Scoring System and Japanese Consensus Group Criteria for Atypical Drug-Induced Hypersensitivity Syndrome (DiHS): A Comparative Analysis.Indian Dermatol Online J. 2022 Jan 24;13(1):40-45. doi: 10.4103/idoj.idoj_196_21. eCollection 2022 Jan-Feb. Indian Dermatol Online J. 2022. PMID: 35198466 Free PMC article.
-
Hemodialysis treatment of vancomycin-induced drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome in a patient undergoing peritoneal dialysis.CEN Case Rep. 2024 Oct;13(5):339-345. doi: 10.1007/s13730-023-00847-x. Epub 2024 Feb 10. CEN Case Rep. 2024. PMID: 38337109 Free PMC article.
-
Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review.Eur J Clin Pharmacol. 2021 Mar;77(3):275-289. doi: 10.1007/s00228-020-03005-9. Epub 2020 Oct 6. Eur J Clin Pharmacol. 2021. PMID: 33025080 Free PMC article. Review.
-
Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS).Int J Mol Sci. 2021 Feb 21;22(4):2147. doi: 10.3390/ijms22042147. Int J Mol Sci. 2021. PMID: 33670052 Free PMC article. Review.
Cited by
-
Vancomycin-Induced DRESS Syndrome: A Systematic Review of Case Reports.Hosp Pharm. 2025 May 23:00185787251341739. doi: 10.1177/00185787251341739. Online ahead of print. Hosp Pharm. 2025. PMID: 40417636 Free PMC article. Review.
-
The diagnosis of acute interstitial nephritis caused by infection versus antibiotic-induced interstitial nephritis: a narrative review.Clin Kidney J. 2024 Mar 4;17(4):sfae054. doi: 10.1093/ckj/sfae054. eCollection 2024 Apr. Clin Kidney J. 2024. PMID: 38572500 Free PMC article. Review.
-
DRESS Mimicking Flushing Syndrome Associated with Vancomycin: A Case Report.Curr Drug Saf. 2025;20(4):514-518. doi: 10.2174/0115748863333025241113055751. Curr Drug Saf. 2025. PMID: 39781732
References
-
- Cabañas R, Calderón O, Ramírez E, Fiandor A, Caballero T, Heredia R, et al. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin Exp Allergy. 2018;48:325–333. - PubMed
-
- Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68:301–308. - PubMed
-
- Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. 2014;69:420–437. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

