A review on the evolution of methods for intestinal in vitro organ culture and its application in veterinary science
- PMID: 37042004
- PMCID: PMC10082705
- DOI: 10.14202/vetworld.2023.347-356
A review on the evolution of methods for intestinal in vitro organ culture and its application in veterinary science
Abstract
Different techniques have been reported in studies of intestinal in vitro organ culture (IVOC). A robust compilation of all available methods is lacking in the literature, making it difficult to choose a method that corresponds to the study's demands. In this review, readers can assess the most available methods, allowing them to evaluate which is more suitable for their purposes and requirements. A simplified view of culturing intestinal explants is presented, highlighting the approachability of IVOC. Relevant findings from diverse veterinarian studies, where explants played a major role, as well as the technique used in each, are described to illustrate its applications. Finally, the strengths and limitations of the innovative intestinal IVOC methods are discussed. This review provides a collection of methods for intestinal explant culture and their possible applications in veterinary research. In this way, it aims to broaden access to IVOC techniques and aid decision-making regarding the best suited for a study's purposes.
Keywords: enteropathogens; explants culture; intestinal pathogens; swine colon; ussing chamber.
Copyright: © Cortez and Guedes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
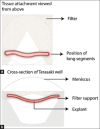
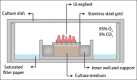
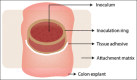
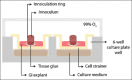

Similar articles
-
In Vitro Porcine (Explant) Colon Culture.Methods Mol Biol. 2024;2749:91-101. doi: 10.1007/978-1-0716-3609-1_9. Methods Mol Biol. 2024. PMID: 38133777
-
In Vitro Porcine Colon Culture.Methods Mol Biol. 2018;1817:185-195. doi: 10.1007/978-1-4939-8600-2_18. Methods Mol Biol. 2018. PMID: 29959714
-
Development and evaluation of a porcine in vitro colon organ culture technique.In Vitro Cell Dev Biol Anim. 2016 Oct;52(9):942-952. doi: 10.1007/s11626-016-0060-y. Epub 2016 Jun 23. In Vitro Cell Dev Biol Anim. 2016. PMID: 27338737
-
A guide to Ussing chamber studies of mouse intestine.Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1151-66. doi: 10.1152/ajpgi.90649.2008. Epub 2009 Apr 2. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19342508 Free PMC article. Review.
-
Explant culture of gastrointestinal tissue: a review of methods and applications.Cell Biol Toxicol. 2011 Aug;27(4):267-84. doi: 10.1007/s10565-011-9187-5. Epub 2011 Mar 8. Cell Biol Toxicol. 2011. PMID: 21384137 Review.
References
-
- Costa M, Hill J, Dame M, Harding J.C.S. In vitro porcine colon culture. In:Methods in Molecular Biology. Humana Press Inc United States. (2018):185–195. - PubMed
-
- Randall K.J, Turton J, Foster J.R. Explant culture of gastrointestinal tissue:A review of methods and applications. Cell Biol. Toxicol. 2011;27(4):267–284. - PubMed
-
- Beck M, Thomson D. Some further research on the cultivation of tissues in vitro. J. R. Soc. Med. 1914;7:21–47.
-
- Trowell O.A. A modified technique for organ culture in vitro. Exp. Cell. Res. 1954;6(1):246–248. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
