Microbial Indicators of Dental Health, Dysbiosis, and Early Childhood Caries
- PMID: 37042041
- PMCID: PMC10288166
- DOI: 10.1177/00220345231160756
Microbial Indicators of Dental Health, Dysbiosis, and Early Childhood Caries
Abstract
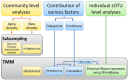
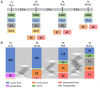
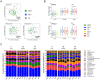
Dental caries lesions are a clinical manifestation of disease, preceded by microbial dysbiosis, which is poorly characterized and thought to be associated with saccharolytic taxa. Here, we assessed the associations between the oral microbiome of children and various caries risk factors such as demographics and behavioral and clinical data across early childhood and characterized over time the salivary and dental plaque microbiome of children before clinical diagnosis of caries lesions. Children (N = 266) were examined clinically at ~1, 2.5, 4, and 6.5 y of age. The microbiome samples were collected at 1, 2.5, and 4 y. Caries groups consisted of children who remained caries free (International Caries Detection and Assessment System [ICDAS] = 0) at all time points (CFAT) (n = 50); children diagnosed with caries (ICDAS ≥ 1) at 6.5 y (C6.5), 4 y (C4), or 2.5 y of age (C2.5); and children with early caries or advanced caries lesions at specific time points. Microbial community analyses were performed on zero-radius operational taxonomic units (zOTUs) obtained from V4 of 16S ribosomal RNA gene amplicon sequences. The oral microbiome of the children was affected by various factors, including antibiotic use, demographics, and dietary habits of the children and their caregivers. At all time points, various risk factors explained more of the variation in the dental plaque microbiome than in saliva. At 1 y, composition of saliva of the C4 group differed from that of the CFAT group, while at 2.5 y, this difference was observed only in plaque. At 4 y, multiple salivary and plaque zOTUs of genera Prevotella and Leptotrichia were significantly higher in samples of the C6.5 group than those of the CFAT group. In conclusion, up to 3 y prior to clinical caries detection, the oral microbial communities were already in a state of dysbiosis that was dominated by proteolytic taxa. Plaque discriminated dysbiotic oral ecosystems from healthy ones better than saliva.
Keywords: 16S rRNA; antibiotics; child; dental caries; dental plaque; saliva.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M. Fontana was a member of the National Scientific Advisory Committee of the Delta Dental Plans Association.
Figures


References
-
- Banting DW, Eggertsson H, Zandoná AGF, Ismail AI, Longbottom C, Pitts NB, Reich E, Ricketts D, Selwitz RH, Sohn W. 2012. Rationale and evidence for the International Caries Detection and Assessment System (ICDAS II) author: International Caries Detection and Assessment System Coordinating Committee [accessed 2023 Feb 14]. https://www.semanticscholar.org/paper/Rationale-and-Evidence-for-the-Int....
-
- Campbell SB, Shaw DS, Gilliom M. 2000. Early externalizing behavior problems: toddlers and preschoolers at risk for later maladjustment. Dev Psychopathol. 12(3):467–488. - PubMed
-
- Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. 2004. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics. 20(1):93–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

