Antibiotic clinical decision support for pneumonia in the ED: A randomized trial
- PMID: 37042682
- PMCID: PMC10247532
- DOI: 10.1002/jhm.13101
Antibiotic clinical decision support for pneumonia in the ED: A randomized trial
Abstract
Background: Electronic health record-based clinical decision support (CDS) is a promising antibiotic stewardship strategy. Few studies have evaluated the effectiveness of antibiotic CDS in the pediatric emergency department (ED).
Objective: To compare the effectiveness of antibiotic CDS vs. usual care for promoting guideline-concordant antibiotic prescribing for pneumonia in the pediatric ED.
Design: Pragmatic randomized clinical trial.
Setting and participants: Encounters for children (6 months-18 years) with pneumonia presenting to two tertiary care children s hospital EDs in the United States.
Intervention: CDS or usual care was randomly assigned during 4-week periods within each site. The CDS intervention provided antibiotic recommendations tailored to each encounter and in accordance with national guidelines.
Main outcome and measures: The primary outcome was exclusive guideline-concordant antibiotic prescribing within the first 24 h of care. Safety outcomes included time to first antibiotic order, encounter length of stay, delayed intensive care, and 3- and 7-day revisits.
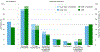
Results: 1027 encounters were included, encompassing 478 randomized to usual care and 549 to CDS. Exclusive guideline-concordant prescribing did not differ at 24 h (CDS, 51.7% vs. usual care, 53.3%; odds ratio [OR] 0.94 [95% confidence interval [CI]: 0.73, 1.20]). In pre-specified stratified analyses, CDS was associated with guideline-concordant prescribing among encounters discharged from the ED (74.9% vs. 66.0%; OR 1.53 [95% CI: 1.01, 2.33]), but not among hospitalized encounters. Mean time to first antibiotic was shorter in the CDS group (3.0 vs 3.4 h; p = .024). There were no differences in safety outcomes.
Conclusions: Effectiveness of ED-based antibiotic CDS was greatest among those discharged from the ED. Longitudinal interventions designed to target both ED and inpatient clinicians and to address common implementation challenges may enhance the effectiveness of CDS as a stewardship tool.
© 2023 Society of Hospital Medicine.
Conflict of interest statement
Derek Williams reports in-kind research support from Biomerieux for unrelated work; Judith Martin receives funding from Merck, Sharp and Dome for unrelated work; Carlos Grijalva reports consultancy fees from Pfizer, Merck, and Sanofi-Pasteur; and grants from Campbell Alliance/Syneos Health and Sanofi for unrelated work. Robert Freundlich reports stock in 3M and consulting from Oak Hill Clinical Informatics for unrelated work.
Figures



Comment in
-
Making tools that work for us: Improving clinical decision support.J Hosp Med. 2023 Jun;18(6):558-559. doi: 10.1002/jhm.13114. Epub 2023 May 1. J Hosp Med. 2023. PMID: 37127944 No abstract available.
References
-
- Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol 2013;34:1252–8. - PubMed
-
- National Estimates on Use of Hospitals by Children from the HCUP Kids’ Inpatient Database (KID). AHRQ, 2012. (Accessed Jan 12, 2014, at http://hcupnet.ahrq.gov/.)
-
- Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of america. Clin Infect Dis 2011;53:e25–76. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

