Clonal hematopoiesis in older patients with breast cancer receiving chemotherapy
- PMID: 37042724
- PMCID: PMC10407695
- DOI: 10.1093/jnci/djad065
Clonal hematopoiesis in older patients with breast cancer receiving chemotherapy
Abstract
Background: The expansion of hematopoietic stem cells carrying recurrent somatic mutations, termed clonal hematopoiesis (CH), is common in elderly individuals and is associated with increased risk of myeloid malignancy and all-cause mortality. Though chemotherapy is a known risk factor for developing CH, how myelosuppressive therapies affect the short-term dynamics of CH remains incompletely understood. Most studies have been limited by retrospective design, heterogeneous patient populations, varied techniques to identifying CH, and analysis of single timepoints.
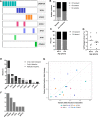
Methods: We examined serial samples from 40 older women with triple-negative or hormone receptor-positive breast cancer treated on the prospective ADjuVANt Chemotherapy in the Elderly trial to evaluate the prevalence and dynamics of CH at baseline and throughout chemotherapy (6 and 12 weeks).
Results: CH was detected in 44% of patients at baseline and in 53% at any timepoint. Baseline patient characteristics were not associated with CH. Over the course of treatment, mutations exhibited a variety of dynamics, including emergence, expansion, contraction, and disappearance. All mutations in TP53 (n = 3) and PPM1D (n = 4), genes that regulate the DNA damage response, either became detectable or expanded over the course of treatment. Neutropenia was more common in patients with CH, particularly when the mutations became detectable during treatment, and CH was significantly associated with cyclophosphamide dose reductions and holds (P = .02).
Conclusions: Our study shows that CH is common, dynamic, and of potential clinical significance in this population. Our results should stimulate larger efforts to understand the biological and clinical importance of CH in solid tumor malignancies.
Trial registration: ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT03858322). Clinical trial registration number: NCT03858322.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
MSS reports institutional funding for research from Novartis, Seattle Genetics, Eli Lilly, and Pfizer, and grant support from the NIH K76AG074918 (Sedrak), R03AG064377 (Sedrak), and R21CA277660 (Sedrak). ELM reports consulting for AstraZeneca, Lilly, Gilead, and Novartis. EAM reports compensated service on scientific advisory boards for BioNTech and Merck; uncompensated service on steering committees for Bristol Myers Squibb and Roche/Genentech; and institutional research support from Roche/Genentech (via SU2C grant) and Gilead. EAM also reports research funding from Susan Komen for the Cure, for which she serves as a Scientific Advisor, and uncompensated participation as a member of the American Society of Clinical Oncology Board of Directors. ASK reports consulting for LabCorp Research and funding from the Multiple Myeloma Research Foundation. RAF reports institutional funding from Eisai. PGM reports consulting fees from Foundation Medicine and Roche. The remaining authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

