Transforming Growth Factor β Signaling Promotes HIV-1 Infection in Activated and Resting Memory CD4+ T Cells
- PMID: 37042759
- PMCID: PMC10231204
- DOI: 10.1128/jvi.00270-23
Transforming Growth Factor β Signaling Promotes HIV-1 Infection in Activated and Resting Memory CD4+ T Cells
Abstract
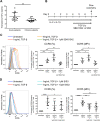
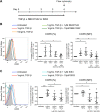
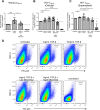
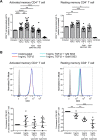
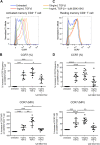
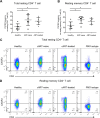
Understanding the facilitator of HIV-1 infection and subsequent latency establishment may aid the discovery of potential therapeutic targets. Here, we report the elevation of plasma transforming growth factor β (TGF-β) during acute HIV-1 infection among men who have sex with men (MSM). Using a serum-free in vitro system, we further delineated the role of TGF-β signaling in mediating HIV-1 infection of activated and resting memory CD4+ T cells. TGF-β could upregulate both the frequency and expression of the HIV-1 coreceptor CCR5, thereby augmenting CCR5-tropic viral infection of resting and activated memory CD4+ T cells via Smad3 activation. The production of live HIV-1JR-FL upon infection and reactivation was increased in TGF-β-treated resting memory CD4+ T cells without increasing CD4 expression or inducing T cell activation. The expression of CCR7, a central memory T cell marker that serves as a chemokine receptor to facilitate T cell trafficking into lymphoid organs, was also elevated on TGF-β-treated resting and activated memory CD4+ T cells. Moreover, the expression of CXCR3, a chemokine receptor recently reported to facilitate CCR5-tropic HIV-1 infection, was increased on resting and activated memory CD4+ T cells upon TGF-β treatment. These findings were coherent with the observation that ex vivo CCR5 and CXCR3 expression on total resting and resting memory CD4+ T cells in combination antiretroviral therapy (cART)-naive and cART-treated patients were higher than in healthy individuals. Overall, the study demonstrated that TGF-β upregulation induced by acute HIV-1 infection might promote latency reservoir establishment by increasing infected resting memory CD4+ T cells and lymphoid organ homing of infected central memory CD4+ T cells. Therefore, TGF-β blockade may serve as a potential supplementary regimen for HIV-1 functional cure by reducing viral latency. IMPORTANCE Incomplete eradication of HIV-1 latency reservoirs remains the major hurdle in achieving a complete HIV/AIDS cure. Dissecting the facilitator of latency reservoir establishment may aid the discovery of druggable targets for HIV-1 cure. This study showed that the T cell immunomodulatory cytokine TGF-β was upregulated during the acute phase of infection. Using an in vitro serum-free system, we specifically delineated that TGF-β promoted HIV-1 infection of both resting and activated memory CD4+ T cells via the induction of host CCR5 coreceptor. Moreover, TGF-β-upregulated CCR7 or CXCR3 might promote HIV-1 latent infection by facilitating lymphoid homing or IP-10-mediated viral entry and DNA integration, respectively. Infected resting and central memory CD4+ T cells are important latency reservoirs. Increased infection of these cells mediated by TGF-β will promote latency reservoir establishment during early infection. This study, therefore, highlighted the potential use of TGF-β blockade as a supplementary regimen with cART in acute patients to reduce viral latency.
Keywords: CCR5-tropic infection; TGF-β; activated memory CD4 T cells; human immunodeficiency virus; resting memory CD4 T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. 2013. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 381:2109–2117. doi:10.1016/S0140-6736(13)60104-X. - DOI - PMC - PubMed
-
- Lee E, Bacchetti P, Milush J, Shao W, Boritz E, Douek D, Fromentin R, Liegler T, Hoh R, Deeks SG, Hecht FM, Chomont N, Palmer S. 2019. Memory CD4 + T-cells expressing HLA-DR contribute to HIV persistence during prolonged antiretroviral therapy. Front Microbiol 10:2214. doi:10.3389/fmicb.2019.02214. - DOI - PMC - PubMed
-
- Tran TA, de Goer de Herve MG, Hendel-Chavez H, Dembele B, Le Nevot E, Abbed K, Pallier C, Goujard C, Gasnault J, Delfraissy JF, Balazuc AM, Taoufik Y. 2008. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One 3:e3305. doi:10.1371/journal.pone.0003305. - DOI - PMC - PubMed
-
- Leite TF, Delatorre E, Cortes FH, Ferreira ACG, Cardoso SW, Grinsztejn B, de Andrade MM, Veloso VG, Morgado MG, Guimaraes ML. 2019. Reduction of HIV-1 reservoir size and diversity after 1 year of cART among Brazilian individuals starting treatment during early stages of acute infection. Front Microbiol 10:145. doi:10.3389/fmicb.2019.00145. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

