Formate Metabolism in Shigella flexneri and Its Effect on HeLa Cells at Different Stages during the Infectious Process
- PMID: 37042762
- PMCID: PMC10269805
- DOI: 10.1128/spectrum.00631-22
Formate Metabolism in Shigella flexneri and Its Effect on HeLa Cells at Different Stages during the Infectious Process
Abstract
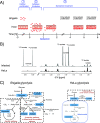
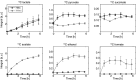
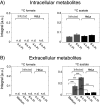
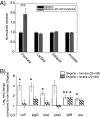
Shigellosis caused by Shigella is one of the most important foodborne illnesses in global health, but little is known about the metabolic cross talk between this bacterial pathogen and its host cells during the different stages of the infection process. A detailed understanding of the metabolism can potentially lead to new drug targets remedying the pressing problem of antibiotic resistance. Here, we use stable isotope-resolved metabolomics as an unbiased and fast method to investigate how Shigella metabolizes 13C-glucose in three different environments: inside the host cells, adhering to the host cells, and alone in suspension. We find that especially formate metabolism by bacteria is sensitive to these different environments. The role of formate in pathogen metabolism is sparsely described in the literature compared to the roles of acetate and butyrate. However, its metabolic pathway is regarded as a potential drug target due to its production in microorganisms and its absence in humans. Our study provides new knowledge about the regulatory effect of formate. Bacterial metabolism of formate is pH dependent when studied alone in culture medium, whereas this effect is less pronounced when the bacteria adhere to the host cells. Once the bacteria are inside the host cells, we find that formate accumulation is reduced. Formate also affects the host cells resulting in a reduced infection rate. This was correlated to an increased immune response. Thus, intriguingly formate plays a double role in pathogenesis by increasing the virulence of Shigella and at the same time stimulating the immune response of the host. IMPORTANCE Bacterial infection is a pressing societal concern due to development of resistance toward known antibiotics. Central carbon metabolism has been suggested as a potential new target for drug development, but metabolic changes upon infection remain incompletely understood. Here, we used a cellular infection model to study how the bacterial pathogen Shigella adapts its metabolism depending on the environment starting from the extracellular medium until Shigella successfully invaded and proliferated inside host cells. The mixed-acid fermentation of Shigella was the major metabolic pathway during the infectious process, and the glucose-derived metabolite formate surprisingly played a divergent role in the pathogen and in the host cell. Our data show reduced infection rate when both host cells and bacteria were treated with formate, which correlated with an upregulated immune response in the host cells. The formate metabolism in Shigella thus potentially provides a route toward alternative treatment strategies for Shigella prevention.
Keywords: Shigella; formate; host-pathogen interactions; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Miethke M, Pieroni M, Weber T, Bronstrup M, Hammann P, Halby L, Arimondo PB, Glaser P, Aigle B, Bode HB, Moreira R, Li Y, Luzhetskyy A, Medema MH, Pernodet JL, Stadler M, Tormo JR, Genilloud O, Truman AW, Weissman KJ, Takano E, Sabatini S, Stegmann E, Brotz-Oesterhelt H, Wohlleben W, Seemann M, Empting M, Hirsch AKH, Loretz B, Lehr CM, Titz A, Herrmann J, Jaeger T, Alt S, Hesterkamp T, Winterhalter M, Schiefer A, Pfarr K, Hoerauf A, Graz H, Graz M, Lindvall M, Ramurthy S, Karlen A, van Dongen M, Petkovic H, Keller A, Peyrane F, Donadio S, Fraisse L, et al. . 2021. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 5:726–749. doi:10.1038/s41570-021-00313-1. - DOI - PMC - PubMed
-
- Hartman HB, Fell DA, Rossell S, Jensen PR, Woodward MJ, Thorndahl L, Jelsbak L, Olsen JE, Raghunathan A, Daefler S, Poolman MG. 2014. Identification of potential drug targets in Salmonella enterica sv. Typhimurium using metabolic modelling and experimental validation. Microbiology (Reading) 160:1252–1266. doi:10.1099/mic.0.076091-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

